Quiz V Ba(NO3)2 Ba2+ + 2NO NaCH3COO H2O Na+ + CH 3COO
advertisement

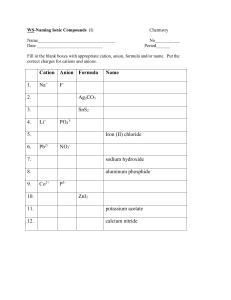
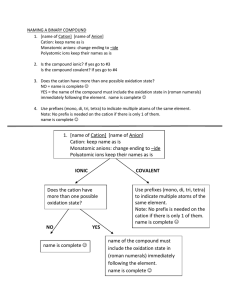
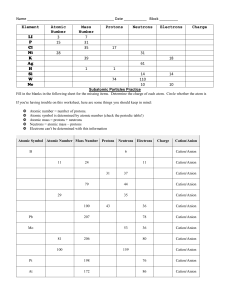
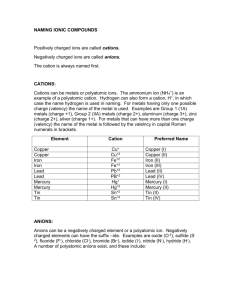
Quiz V 1) Label the following salts as neutral, acidic, or basic and write down the reactions that control the acid-base behavior: Ba(NO3)2, NaCH3COO, NH4 Cl, K2SO4. 2O Ba(NO 3 ) 2 "H" # Ba 2+ + 2NO$3 From the above, we have the cation of a strong base, Ba(OH) 2, and the anion of a strong acid, HNO3. Therefore, Ba(NO3)2 is neutral. ! 2O NaCH 3COO "H" # Na + + CH 3COO$ CH 3COO$ + H 2O % CH 3COOH + OH $ Na+ is the cation of a strong base, NaOH, and acetate is the anion of the weak acid acetic acid. Thus, NaCH3 COO is a weak base. ! 2O NH 4 Cl "H" # NH +4 + Cl$ NH +4 + H 2O % NH 3 + H 3O + Ammonium is the cation of a weak base, NH3, and chloride is the anion of a strong acid, HCl. NH4Cl must be a weak acid. ! 2O K 2SO 4 "H" # 2K + + SO 4 2$ 2$ $ SO 4 + H 2O % HSO 4 + OH $ ! Potassium is the cation of a strong base, sulfate is the anion of a weak acid , HSO4- . K2SO4 is therefore a weak base. This next part was not necessary on the quiz, but to convince you of this let’s look at it a bit closer. The Ka of the hydrogen sulfate ion is 1x10-2, so if one dissolved enough K2SO4 to make a 1.0 M solution the pH would be: K w = K aK b K b = K wK a = 10"14 M 2 /10"2 = 10"12 M Kb = [HSO"4 ][OH" ] [SO 2" 4 ] 10"12 M = x([OH" ]o + x) x(10"7 M + x) x2 = # [SO 2" 1.0M " x 1.0M 4 ]o " x x = [OH" ] = 3.2x10"6 [H + ][OH" ] = 10"14 [H + ] = 3.2x10"9 pH = 8.5, which is greater than 7 so the solution is basic ! 2) Consider two different acids, HA and HB, with the following acidities: pKa(HA) = 4 and pKa(HB) = 5. What concentration ratios are needed to make a buffer with pH = 4.8? Which of the two buffers has the higher capacity? Explain. To do this problem, make use of the Hendersen-Hasselbach equation. [base] [acid] [base] pH " pK a = log [acid] [base] = 10(pH"pK a ) [acid] pH = pK a + log For a pH of 4.8, and pK a of 4 and 5 [A] = 10(4.8"4) = 6(.3) [HA] [B] ! = 10(4.8"5) = 0.6(3) [HB] Where the parentheses indicate a non - significant digit HB will have a higher capacity because the ratio above is closer to one, or equivalently because its pKa is closest to the desired pH. !