Practice #1 KEY
advertisement

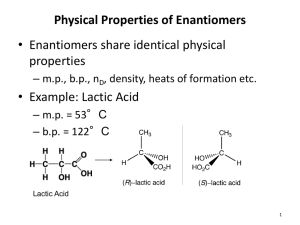
CHE 230 Practice Problems 1 1. Draw the structures of the following compounds. Be sure to indicate the relevant stereochemistry. S –1-Bromo-1-chloroethane. Br Br Cl Cl CH3 H CH3 R-2-butanol OH 2. Draw the molecular formula for an unsaturated alkyl chloride (C5H9Cl) that shows a) neither optical activity or E,Z isomerism Cl b) both optical activity and E, Z – isomerism Cl c) E, Z, isomerism but not optical activity Cl d) optical activity but not E, Z isomerism. Cl . Draw all of the stereoisomers of 2,3-butandiol. Label meso compounds and pairs of enantiomers. OH OH OH OH OH OH Enantiomers OH Meso OH 4. What is the relationship between the structures in each pair? Are they the same compounds, drawn from different perspectives? Are they conformational isomers? Are they enantiomers? Are they diastereomers? Are they enantiomers drawn in different conformations? Enantiomers. In planar projections these are: Same Compound (drawn from different sides) Enantiomers (enantiomers of trans-1,2-diethylcyclohexane, drawn in different conformations) O Br OCH3 O Br Diastereomers (differ at 2 of 3 stereogenic centers) OCH3 5. How many stereoisomers are there of the allylic alcohol below? (think carefully about the meaning of the term “stereoisomer”) OH CH2CH3 There are 2 stereogenic centers, so there are 4 possible stereoisomers based on R,S differences. However, there is also a C=C bond, and there is the possiblility of an E and a Z isomer of each of the 4 R,S – based stereoisomers. This leads to a total of 8 possible stereoisomers. E,R,R Z,R,R E,R,S Z,R,S E,S,R Z,S,R E,S,S Z,S,S How about for adamantanol? OH There are only 2 stereoisomers (enantiomers).