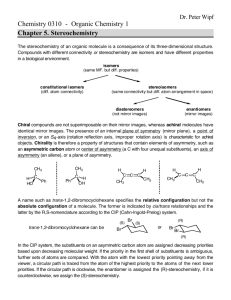
enantiomers
advertisement
Experiment 9: Stereochemistry: Enantiomers and Diastereomers Objectives To learn how to identify stereocenters using molecular models. To practice assigning the absolute configuration of stereoisomers using the R-S convention. To learn to identify the stereochemical relationship between stereoisomers. To learn how to use Fischer projections. Chirality ACHIRAL If an object has a plane of symmetry, such as this flask, it is identical to its mirror image. CHIRAL The lack of a plane of symmetry is called “handedness”, or chirality, such as this hand. Its mirror images are NOT identical. Chirality A point in a molecule where four different groups (or atoms) are attached to carbon is called a chirality center Enantiomers When H and OH substituents match up, COOH and CH3 don’t when COOH and CH3 coincide, H and OH don’t Enantiomers vs. Diastereomers Molecules with more than one chirality center have mirror image stereoisomers that are enantiomers In addition they can have stereoisomeric forms that are not mirror images, called diastereomers Diastereomers Enantiomers 2R,3R 2R,3S 2S,3S 2S,3R Enantiomers Meso Forms Tartaric acid has two chirality centers and two diastereomeric forms An achiral compound with chirality centers is called a meso compound – it has a plane of symmetry The two structures on the right in the figure are identical so the compound (2R, 3S) is achiral MESO Fischer Projections oThe conversion of a perspective drawing to a Fischer projection requires rotating the molecule so that the “top” and “bottom” groups are oriented back, away from you as is shown in the molecule below. Press flat W X Y Z Z Z C W C Y X W C Y X Fischer Projections And another example… Stereochemical Relationships Diastereomers Diastereomers CH3 CH3 Cl H H OH H Cl Cl H H Cl HO H HO H H OH CH3 CH3 "A" CH3 CH3 "B" Enantiomers CH3 "C" CH3 "D" Enantiomers Diastereomers Diastereomers Enantiomers and Meso Forms COOH COOH H HO OH H COOH 2R, 3R HO H COOH COOH H H OH HO H OH H OH HO H COOH 2S, 3S COOH 2R, 3S COOH 2S, 3R Look closely at these two… There is a plane of symmetry. The top half of the molecules is identical to the bottom half! The plane of symmetry makes them achiral, although they do have chiral centers. Identifying Stereochemical Relationship Identify stereocenters. Assign absolute configuration to each stereocenter. Determine stereochemical relationship based on absolute configurations. ENANTIOMERS Different absolute configuration at ALL stereocenters DIASTEREOMERS Same absolute configuration at SOME stereocenters, different at some. Physical Properties of Stereoisomers Enantiomers: SAME: mp, d, solubility. DIFFERENT: direction of rotation of plane-polarized light. Diastereomers : DIFFERENT: mp, d, solubility, AND direction of rotation of plane-polarized light. EXPERIMENTAL PROCEDURE In today’s experiment, molecular models will be used to: Practice identification of chiral centers. Assign priority to groups attached to chiral centers based on the CIP rules. Assign absolute configuration of chiral centers. Convert 3d structures to 2d Fischer projections. Identify stereochemical relationships between isomers.