Document
advertisement

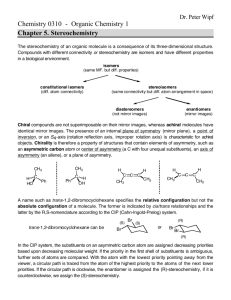
Physical Properties of Enantiomers • Enantiomers share identical physical properties – m.p., b.p., nD, density, heats of formation etc. • Example: Lactic Acid – m.p. = 53°C – b.p. = 122°C Lactic Acid 1 • Enantiomers rotate a plane of polarized light in equal, but opposite directions – Called “Optical Activity” – Use Polarimeter 2 Optical Activity • If the sample rotates the plane of polarized light CW → dextrorotatory (+) – Latin: Dexter = Right • If the sample rotates the plane of polarized light CCW → levorotatory (-) – Latin = Laevus = Left • If one enantiomer is +, the other will be – (+)-lactic acid (–)-lactic acid 3 • The sign of optical rotation is unrelated to R and S configuration of a compound (S)-(+)-lactic acid (R)-(–)-lactic acid • Optical rotation (a) is a quantitative measure of optical activity [a] = a/cl [a] = specific rotation a = degrees c = concentration (g/mL) l = path length (dm) Often, temp and wavelength indicated (R)-(+)-glyceraldehyde [α]20 = +13.5° mL g-1 dm-1 (S)-(-)-glyceraldehyde [α]20 = -13.5° mL g-1 dm-1 Problems 1) A 1.50 g sample of (S)-(+)-coniine, the toxic extract of poison hemlock, was dissolved in 10.0 mL of ethanol and placed in a sample cell with a 5.00 cm pathlength. The observed rotation at the sodium D line was +1.21°. Calculate the [α]D for coiine. 2) What will the specific rotation be for the R enantiomer? 7 Racemic Mixtures • Racemic mixture/Racemate: a mixture containing equal amounts of two enantiomers – typically have different physical properties from that of the pure enantiomers – Example: Lactic Acid • m.p. (R or S) = 53°C • m.p. (R and S)= 17°C – Indicating racemic mixture: • Racemic Lactic Acid • (±)-Lactic Acid – Optical rotation = 0 6.4 Racemates 8 Making Racemic Mixtures • Racemization: the process of forming a racemic mixture – Easy: mix = amounts of each enantiomer together – More difficult: chemical rxns • Heat, enzymes, acids, bases Celexa vs. Lexapro R-(−)-citalopram • Celexa = racemic mixture • Lexapro = enantiopure S – escitalopram S-(+)-citalopram Resolution of Enantiomers • Enantiomeric resolution: the separation of a pair of enantiomers from a racemic mixutre – Also called chiral resolution, optical resolution, and mechanical resolution – Often difficult • Enantiomers have same chemical properties • Separation often through –Selective crystallization –Diastereomeric salt formation Selective Crystallization • Pasteur’s experiment (1848) • 1882 – “Seeding” of a supersaturated solution – Now often used in industry • Methadone • L-glutamic acid Diastereomers • Diastereomers/Diastereoisomers: stereoisomers that are not mirror images (enantiomers) of each other – possible when a molecule has two or more asymmetric carbons – Differ in all their physical properties 6.6 Diastereomers 14 Isomer Identification Flowchart 6.6 Diastereomers 15 • Molecules with more than one chirality center have mirror image stereoisomers that are enantiomers • In addition they can have stereoisomeric forms that are not mirror images, called diastereomers Diastereomers and Enantiomers 6.6 Diastereomers 17 Problem: Can you identify the diastereomers? 2R,3R 2S,3S 2R,3S 2S,3R 19 Physical Properties of Stereoisomers 6.6 Diastereomers 20