test#2 blank

Name_______________
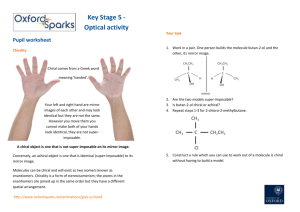
1) Define all chiral centers as either R or S. (12 Points)
Organic Chemistry
Test #2
100 Points
10/17/14
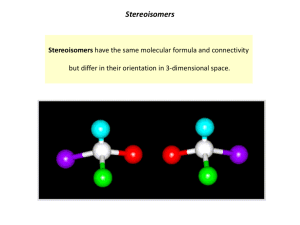
2) Define the following pairs of molecules as Diastereomers, Enantiomers or the same. (9 points)
1
3) Synthesize 2R 2-pentanol from 2R 2-Bromopentane. You can use any other reagents you want. (7 points)
4) How many stereoisomers are possible for the following molecule? (4 points)
5) Draw the enantiomer and a diastereomer of the following molecule. (6 points)
2
6) A mixture of two enantiomers (below) were looked at in a polarimeter. It read
+12 o
[
] S-3-hexanol = +24
0
[
] R-3-hexanol = -24
0 a) What was the enantiomeric excess of this solution? (4 points) b) What is the total percentage of each enantiomer? (4 points)
7) Draw both chair forms of trans-1,2-dibromocyclohexane (5 points)
8) Circle the structure in question 7 that has the lowest energy. (2 points)
9) For each structure: a) Put an asterisk (*) beside each chiral carbon. (5 points) b) Is the molecule chiral, non-chiral or meso? (6 points)
3
9) Draw the mechanism and the product for the following reaction. . (Make sure your arrows are pointed exactly where they should be and the you include all lone pairs of electrons) (5 points)
10) Design a synthesis of (R) 2-methoxy heptane from (S) 2-chloroheptane. (You can use any other reagents you need). (5 points)
11) Circle the solvent(s) from the selection below which would be considered protic
(6 points)
4
12) Which of the following reactions would go faster A or B? Explain Why. (5 points)
13) Fill-in the appropriate reagent, product, starting material (12 points)
14) Define the following (3 points)
Nucleophile –
Electrophile -
5