S - Faculty
advertisement
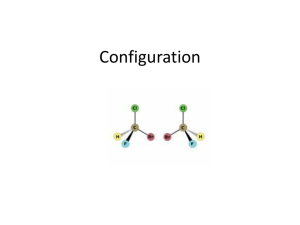
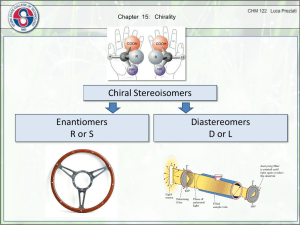
STEREOCHEMISTRY Dr. Clower CHEM 2411 Spring 2014 McMurry (8th ed.) sections 5.1-5.12, 7.5 Stereochemistry • Branch of chemistry concerned with the 3D arrangement of atoms in molecules • Stereoisomers: • Same molecular formula • Same connectivity • Different 3D orientation (cannot be converted via bond rotation) • Previously: • Cis and trans • Now: • Stereochemistry at tetrahedral centers • Enantiomers, diastereomers • E and Z Chirality • “handedness” • Has a mirror image that is nonsuperimposable • Example: hand Chirality • A molecular example: • Try this with your model kit Enantiomers • Chiral molecules form enantiomers • Nonsuperimposable mirror images • Result from tetrahedral C (sp3) with 4 different substituents • This C is called a chirality center (or stereocenter, or asymmetric center) and are often marked with * Identify the chirality centers Achiral molecules • Are superimposable on their mirror images • Contain a plane of symmetry • Cuts through the middle of the molecule so that one half reflects the other half achiral chiral Molecule Stereocenter? Plane of Symmetry? Chrial? Molecule Stereocenter? Plane of Symmetry? • Not all molecules with stereocenters are chiral! Chrial? Enantiomer Similarities and Differences • Same molecular formula, connectivity • Different 3D arrangement • Same physical properties (mp, bp, solubility) • Same spectroscopic properties (IR, NMR, etc.) • Same reactivity, in general • Products will have different stereochemistry • Only one will react with an enzyme (like a hand fitting in a glove) • Different designations (R vs. S) • Different optical activity Optical Activity • Rotation of plane-polarized light; seen in chiral molecules • a = degree of rotation; measured by the polarimeter • One enantiomer rotates light to the left a degrees • Levorotatory (-) • The other rotates light to the right a degrees • Dextrorotatory (+) Optical Rotation • Depends on polarimeter pathlength (l) and sample concentration (c) • Specific rotation [a]D is observed under standard conditions • l = 589.6 nm • l = 1 dm (10 cm) • c = 1 g/cm3 • (-)-Lactic acid has a [a]D of -3.82 • (+)-Lactic acid has a [a]D of +3.82 • What is [a]D of a 50:50 mixture of (-) and (+)-lactic acid? R and S designations • Used to describe 3D configuration about a chirality center • Not related to direction of optical rotation (+) and (-) • To designate R and S need to assign priorities to each group bonded to the stereocenter • Cahn-Ingold-Prelog convention Priority Rules 1. Higher atomic number (of atom bonded to C*) = higher priority -Br > -Cl > -OH > -NH2 > -CH3 > -H 2. If 2 of the same atom are bonded to C*, look at atomic number of the next set of atoms • Continue process until first point of difference • Some more examples: Priority Rules 3. Atoms in double bonds count twice; atoms in triple bonds count three times Which substituent has the higher priority? a) -Br -Cl b) -CH2CH3 -CH(CH3)2 c) -CH=CH2 -CH2CH3 d) -CHO -CO2H e) -CH2OH -CH2CH2OH To designate R or S: 1. Locate chirality center 2. Assign priority to the 4 groups (1 = highest; 4 = lowest) 3. Orient molecule so substituent 4 is point away from you (with model or on paper) 4. Read the other groups 1→2→3 (draw arrow on paper) 5. Groups read clockwise = R; counterclockwise = S Example: 2-bromobutane Example: 2-bromobutane Rank the following groups in order of priority from highest (1) to lowest (4): -NHC(O)CH3 -OCH3 -OH -F Draw R and S stereoisomers for 2-hydroxypropanal: R and S stereoisomers for 3-methylhexane: • Hints: • Switch any two groups to draw the enantiomer • When substituent 4 is forward, 1→2→3 clockwise is S Classify these as chiral or achiral: How many chirality centers? Rotating a Tetrahedral Carbon • To rotate a carbon and not accidentally change the R/S designation, keep one substituent in the same place, and rotate the other three. • Make sure all three groups are rotating in the same direction • Do not switch two groups; this changes the R/S designation Classify these molecules as R or S: Determine whether the two structures in each pair represent constitutional isomers, enantiomers, or identical compounds. a) b) Fischer Projections • Another way of drawing tetrahedral carbons • Horizontal lines = out of page • Vertical lines = into page • Frequently used for chirality centers, especially if a molecule has more than one chiral center What is the relationship between these two molecules? Molecules With Multiple Stereocenters • Maximum # stereoisomers = 2n where n = # stereocenters # Stereocenters # Stereoisomers Stereoisomers 1 2 R S 4 (R,R) (S,S) (R,S) (S,R) 2 Example: 2,3-Pentanediol • Draw Fischer projections for the 4 stereoisomers • Carbon chain vertical, C1 at top Relationships • A and B are enantiomers • C and D are enantiomers • A and C, A and D, B and C, B and D are diastereomers Diastereomers • Stereoisomers that are not mirror images of each other • Different physical properties • With tetrahedral carbons, require at least 2 stereocenters • Cis-trans stereoisomers are also diastereomers Meso Compounds • Maximum # stereoisomers = 2n where n = # stereocenters • The # stereoisomers will be less than 2n when there is a meso compound • Meso compound • An achiral compound which contains chirality centers • Not optically active • The chirality centers typically are identical (have the same 4 substituents) and reflect each other in a plane of symmetry • Example: Another Example: 2,3-Butanediol • A = (2R,3R)-2,3-butanediol • B = (2S,3S)-2,3-butanediol • C = D = meso-2,3-butanediol • C and D are superimposable mirror images (the same molecule) • Relationship between enantiomers and meso? • Diastereomers Racemic Mixtures • aka Racemate, + pair, or d,l pair • 50% mixture of two enantiomers • Not optically active • Separation of enantiomers is difficult • React with chiral compound to convert to a pair of diastereomers which can be separated by distillation, recrystallization, etc. • Separate on chiral column • Separate with enzyme Applications of Stereochemistry 1. Stereochemistry of reactions • If a product has a stereocenter, is the stereochemistry all R, all S, or a mixture? • To understand details, need to look at mechanism (next) Applications of Stereochemistry 2. Reactions with enzymes • Receptors/enzymes react with only one enantiomer (like a handshake) • Limonene • R = orange odor • S = pine odor • Ibuprofen • R = inactive • S = active • D-Decalactone • R = porcupine emits to alert predators • S = coconut Thalidomide • How many chirality centers? • How many stereoisomers? • How was the drug administered? • What effect did this have on patients who used thalidomide? Francisco Goya Alkene Stereochemistry • Previously, cis-trans stereoisomers • Now, E,Z-designation of alkenes • Use E,Z instead of cis-trans when • More than two substituents on C=C • Heteroatoms on C=C • To assign E or Z: • Rank the two groups on each carbon of the C=C according to the Cahn-Ingold-Prelog priority rules • If the higher priority groups are on the same side of the C=C, the alkene has Z geometry • If the higher priority groups are on opposite sides of the C=C, the alkene has E geometry E and Z Configurations Name these alkenes: a) b) Next… • Organic reactions