chiral - Facultypages.morris.umn.edu
advertisement

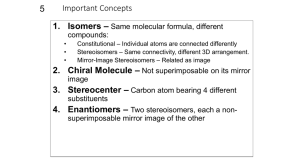
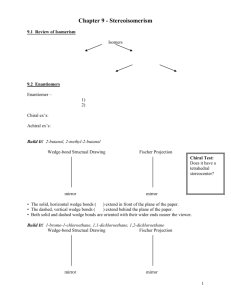
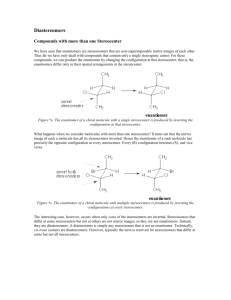
constitutional isomers: conformational isomers: (note – many slides from Soderberg text) stereoisomers: intro Stereoisomers 3.3 Definitions stereoisomers: different arrangement of atoms in space enantiomers: mirror images diastereomers: not mirror images (eg. cis/trans alkene) chiral: not superimposible on mirror image, no plane of symmetry asymmetric center: tetrahedral atoms with 4 different subs stereocenter: exchange two bonds, get different stereoisomer (eg. alkene) enantiomers: mirror images not superimposable 3.3 two enantiomers of thalidomide thalidomide is chiral 3.3 more examples of enantiomers all are chiral if it has an asymmetric center, it is almost certainly chiral (exception – meso) 3.3 achiral molecules (no stereocenters!) wedges don’t necessarily mean a stereocenter, and vice-versa! 3.3 other atoms can be stereocenters Don’t worry about threo / erythro definition 3.3 3.3 stereocenters? asymmetric centers? chiral? The Cahn-Ingold-Prelog system 3.4 3.4 3.4 (effective stereoisomer) what if H is drawn pointing back? 3.4 commercial thalidomide sold as racemic mixture 3.5 proteins recognize stereochemistry! 3.5 other examples of enantiomers with different biological activity but . . . enantiomers have identical physical properties! (except optical rotation, next) determining stereochemistry: optical activity l in dm c in g/mL 3.6 enantiomers have equal but opposite specific rotations racemic mixtures: optically inactive enantiomeric excess eg. if you have 75% R and 25% S, ee = 50% molecules with more than one stereocenter (mirror images) 3.7A notice: diastereomers are not mirror images 3.7A enantiomers: all asymmetric centers different diastereomers have different physical properties different optical rotation at least one, but not all asymmetric centers different cis/trans alkenes are diastereomers – but not source of chirality! (show model) 2n stereoisomers n = # asymmetric centers + # asymmetric alkene groups eg. n = 3 8 total stereoisomers (including this one) OH OH what is the enantiomer of this molecule? R,R,E 3.7A Naming chiral compounds meso compounds rings – look for mirror plane! 3.7B Fischer and Haworth projections (looking down from above) 3.8 ways of drawing open chain form of glucose: 3.8 determining R/S on Fischers convenient to compare sugars: 3.8 Haworth projections (used for sugars in cyclic form) (not in Bruice) 3.8 Determining the absolute configuration of (-)glyceraldehyde (+)-tartaric acid – configuration determined by x-ray crystallography bonds to asymmetric center not broken – (-) GA must be S! stereochemistry and organic reactions (don’t worry about stereospecific vs. stereoselective) racemic mix of enantiomers 2 new centers formed – 4 isomers formed anti addition enzymatic reactions are stereospecific water adds with stereospecificity (enzyme reactions are stereospecific) substrate stereoselectivity: 3.9