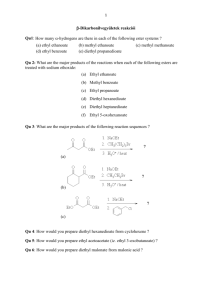
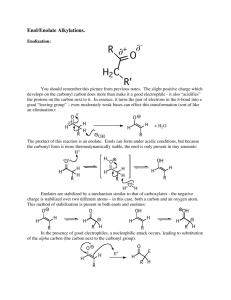
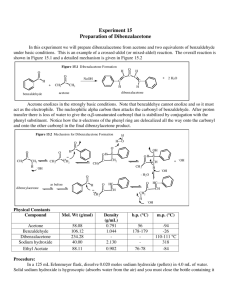
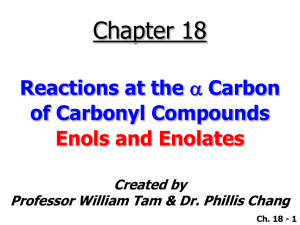
Text Related to Segment 20.05 ©2002 Claude E. Wintner We now
advertisement

Text Related to Segment 20.05 ©2002 Claude E. Wintner We now may return profitably to the previously introduced addition/substitution dichotomy of carbonyl chemistry. In basic medium the aldol condensation is the result of addition of an enolate anion to the carbonyl group of an aldehyde or ketone. Since it is a general property of esters that they undergo instead substitution at the carbonyl functionality, we also may expect to see such a substitution reaction involving an enolate anion. Indeed, under appropriate conditions this is readily observed, and termed the Claisen condensation or, simply, the ester condensation. When two molecules of ethyl acetate are treated with a full mole of sodium ethylate in ethanol, followed by an acidic workup, ethyl acetoacetate (so-called "acetoacetic ester") is produced. Although the pKA of ethyl acetate is 22, and that of ethanol is only 18, sodium ethylate nevertheless is a strong enough base to produce a concentration of the ethyl acetate enolate anion requisite for addition to other molecules of ethyl acetate at their carbonyl groups at a reasonable rate. In turn, this reaction is followed by displacement of ethylate anion from the addition complex, the result being a β-keto ester. Herein lies the crux for the success of the Claisen condensation, allowing it to be driven forward to completion under circumstances that otherwise would be thermodynamically unfavorable: the pK A of ethyl acetoacetate at the carbon atom doubly activated by the two carbonyl groups is only 10. In other words, ethyl acetoacetate is more acidic than is ethanol, by 8 orders of magnitude. Hence, given the full mole of sodium ethylate employed in the reaction, the ethyl acetoacetate effectively is removed from the reaction as soon as it is produced, being transformed instead to its corresponding stable delocalized conjugate anion, the five-orbital-six-electron system shown in the figure: 1 full mole O O H 3C OEt H O EtO Na CH2 EtOH OEt two molecules pK = 22 A of ethyl acetate O H 3C pKA = 18 C H2 pKA = 10 Claisen (Ester) Condensation O EtO Na O workup in acid C OEt H2 ethyl acetoacetate, often called "acetoacetic ester" O C H O OEt OEt H 3C C H O OEt H 3C O C H formation of delocalized anion condensation + elimination = substitution O C H O mechanism: EtO O driving force for the reaction is the formation of the delocalized anion: a five-orbital-six-electron system functionality: -keto ester H 3C O H 3C H 3C O OEt OEt EtO H 2C ethyl acetate H 3C H 3C elimination (overall, substitution) enolate formation enolate EtO EtO O H H O H H EtO H 3C O condensation EtO O H 3C second molecule of ethyl acetate the Claisen or ester condensation, illustrated for ethyl acetate O O The above analysis explains why a full mole of base is necessary to run the Claisen condensation, as well as why this condensation reaction cannot be carried out in acid, where the final driving force cannot exist. As the β-hydroxy carbonyl or α,β -unsaturated carbonyl functionalities are distinguishing indications of the aldol condensation, so is the β-keto ester functionality the hallmark of the Claisen condensation. And, just as for the aldol condensation, so also for the Claisen: intramolecular reaction will lead to cyclic molecules, here cyclic βketo esters. The cyclic Claisen condensation, generally referred to as the Dieckmann condensation, is exemplified by the cyclization of diethyl heptanedioate to form 2carboethoxy cyclohexanone. Again, the difference between the cyclic and acyclic cases simply amounts to a tether — here of three methylene groups — joining the two ethyl acetate moieties of the acyclic case: H2 C H 2C 1) 1 full mole CH2 O H 2C O H 2C OEt OEt new C—C bond EtO Na in EtOH 2) workup in acid O EtO Dieckmann product (functionality: -keto ester) (2-carboethoxy cyclohexanone) diethyl heptanedioate O mechanism: EtO H 2C H2 C H EtO H 2C EtO H2 C O C H2 O H H 2C O H 2C EtO C H2 O product (via delocalized anion and subsequent acid workup) the Dieckmann condensation, illustrated for diethyl heptanedioate ©2002 Claude E. Wintner