Project Overview
advertisement

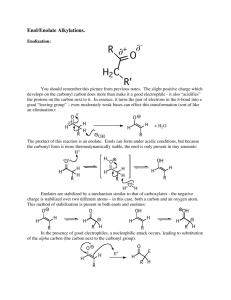
Chapter 18 Reactions at the a Carbon of Carbonyl Compounds Enols and Enolates Created by Professor William Tam & Dr. Phillis Chang Ch. 18 - 1 About The Authors These PowerPoint Lecture Slides were created and prepared by Professor William Tam and his wife, Dr. Phillis Chang. Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Guelph (Ontario, Canada) in 1998 and is currently a Full Professor and Associate Chair in the department. Professor Tam has received several awards in research and teaching, and according to Essential Science Indicators, he is currently ranked as the Top 1% most cited Chemists worldwide. He has published four books and over 80 scientific papers in top international journals such as J. Am. Chem. Soc., Angew. Chem., Org. Lett., and J. Org. Chem. Dr. Phillis Chang received her B.Sc. at New York University (USA) in 1994, her M.Sc. and Ph.D. in 1997 and 2001 at the University of Guelph (Canada). She lives in Guelph with her husband, William, and their son, Matthew. Ch. 18 - 2 Reactions at the a Carbon of Carbonyl Compounds: Enols and Enolates O O Nu R R' R R' Nu O R' R H a Hydrogens are weakly acidic (pKa = 19 – 20) Ch. 18 - 3 1. The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions H H C pKa C H 25 C H pKa 50 H 44 O H H3C C H2C H R' R H 19-20 Ch. 18 - 4 O H C C R B: O O C R C C C R Resonance structures for the delocalized enolates Ch. 18 - 5 O H+ H+ C C R Enolate O HO C C R Enol form H C R Keto form Ch. 18 - 6 2. Keto and Enol Tautomers Interconvertible keto and enol forms are called tautomers, and their interconversion is called tautomerization Ch. 18 - 7 Acetaldehyde Keto form O Enol form OH H (~100%) H (extremely small) O OH Acetone (>99%) O (1.5 X 10-4%) OH Cyclohexanone (98.8%) (1.2%) Ch. 18 - 8 O O OH Pentane-2,4-dione (24%) O Enol form (76%) Hydrogen bond O Resonance stabilization of the enol form H : :O: : : :O H :O: Ch. 18 - 9 3. Reactions via Enols & Enolates 3A. Racemization OH O t Bu Et H Me (chiral) (s) Racemization at an a carbon takes place in the presence of acids or bases OH or H3O t Et Bu Enol (achiral) Me H3O O t O Et Bu H Me + t Et Bu Me ( 1 : 1 ) racemate H Ch. 18 - 10 Base-Catalyzed Enolization H O O C HO C C OH Enolate (achiral) C H O HO + C C H Enol (achiral) Ch. 18 - 11 Acid-Catalyzed Enolization O O C H C + H O C H H H + C H O H H H O H H + O C H C Enol (achiral) Ch. 18 - 12 3B. Halogenation at the a Carbon H O C C + X2 acid or base X O C C + HX (racemic) Ch. 18 - 13 Base-Promoted Step 1 :O: H B: + C Halogenation slow B:H + C C C :O: Enolate fast OH B: + X Step 2 :O: C C Enolate anion C X O C fast + C X C Enol :O: + X Ch. 18 - 14 Acid-Promoted Halogenation Step 1 :B :O: H C H + H:B C O C fast H C O C slow C H + H:B Enol Step 2 X X O + C C H X fast C O C H + X Step 3 X C O C H + X fast :O: X C C Racemic + HX Ch. 18 - 15 3C. The Haloform Reaction O O 3 X2 CX3 3 OH + 3X OH O + CHX3 A haloform (X = Cl, Br, I) O Ch. 18 - 16 O R A methyl ketone O I2, HO (Both in excess) + R O CHI3 Iodoform (a yellow precepitate) Ch. 18 - 17 Mechanism O O H + B R O X Repeat steps O R CX3 twice R Enolate R X O R X + X Ch. 18 - 18 ● Acyl Substitution Step R O : : O :OH CX3 R O CX3 OH R OH + :CX3 HO O A haloform + R : : CHX3 O: Carboxylate anion Ch. 18 - 19 3D. a-Halo Carboxylic Acids: The Hell–Volhard–Zelinski Reaction 1. X2, P O R OH 2. H2O O R OH X Ch. 18 - 20 Example O O OH Br2 Br P Br H2O O OH Br Ch. 18 - 21 O R O P + Br2 OH R [PBr3] :O O Br R Br Br R Br H Br Br O H2O R OH Br Ch. 18 - 22 O N Br (NBS) O HBr, SOCl2 O R Br O R Cl Cl O I2 R Cl HI, SOCl2 I Ch. 18 - 23 1. HO 2. H3O R OH OH a-Hydroxy acid O R O OH X O NH3 R O NH3 a-Amino acid Ch. 18 - 24 4. Lithium Enolates O O + EtO Na H weaker acid (pKa = 19) weaker base O + EtOH stronger base stronger acid (pKa = 16) O + iPr2N Li H weaker acid (pKa = 19) stronger base + iPr2NH weaker base weaker acid (pKa = 38) Ch. 18 - 25 Preparation of lithium diisopropylamide H (LDA) Li Buyllithium (BuLi) + Diisopropylamine (pKa = 38) THF + Butane (pKa = 50) N Li N Lithium diisopropylamine i [LDA or LiN( Pr)2] Ch. 18 - 26 4A. Regioselective Formation of Enolates Formation of a Kinetic Enolate O H3C O Li H H Li N(iPr)2 H3C DME This enolate is formed faster because the hindered strong base removes the less hindered proton faster. Kinetic enolate Ch. 18 - 27 Formation of a Thermodynamic Enolate This enolate is more stable because the double bond is more highly substituted. It is the predominant enolate at equilibrium. B H3C H O Kinetic (less stable) enolate H H H3C O O H H 2-Methylcyclohexanone H3C weak base in a protic Thermodynamic solvent (more stable) enolate Ch. 18 - 28 4B. Direct Alkylation of Ketones via Lithium Enolates O H3C O Li O CH3 I (- LiI) (56%) LDA DME O Br Ph Ph (- LiBr) (42-45%) Ch. 18 - 29 4C. Direct Alkylation of Esters O R LDA OR' THF O R OR' H E O R OR' E Ch. 18 - 30 Examples O O 1. LDA, THF OMe 2. MeI OMe Me O O O 1. LDA, THF 2. Ph O Ph Br Ch. 18 - 31 5. Enolates of b-Dicarbonyl Compounds O O H pKa = 9-11 (more acidic) O H pKa = 18-20 Ch. 18 - 32 Recall O O + EtO + EtOH H a-hydrogens of b-dicarbonyl compounds are more acidic O O O + EtO H O + EtOH Ch. 18 - 33 Contributing resonance structures O O O O O O C C C C C C C C O O C C C C Resonance hybrid Ch. 18 - 34 6. Synthesis of Methyl Ketones: The Acetoacetic Ester Synthesis O O O EtO Na O Na OEt O O OEt t O BuO K R O OEt X OEt R R R' X O O (R, R' = 1o OEt R R' alkyl groups) Ch. 18 - 35 Synthesis of monosubstituted methyl ketones O O O 1. EtO Na , EtOH OEt 2. Ph O OEt Br Ph 1. NaOH O heat O (- CO2) Ph (Decarboxylation of b-keto acid) 2. H3O+ O OH Ph Ch. 18 - 36 Synthesis of disubstituted methyl ketones O O O 1. EtO Na , EtOH OEt O 2. MeI OEt Me 1. tBuOK, tBuOH O O 1. NaOH OH Me O 2. H3O+ 2. Et-Br OEt Me Et heat O Et O Et (- CO2) Me Ch. 18 - 37 O O Ethyl acetoacetate ion is the synthetic equivalent of O Acetate enolate Ch. 18 - 38 O Synthesis of g-keto acids and g-diketones O O EtO Na O OEt O OEt Br O O 1. NaOH (aq) O X O 2. H3O+ OH OEt O X heat (- CO2) O O g X a b X O X=OH: g-keto acid X=R: g-diketone Ch. 18 - 39 6A. Acylation Synthesis b-diketones O NaH DMF O O O OEt (cannot use EtOH because it will react with acid chloride) O O R R 1. NaOH (aq) OH O heat (- CO2) O OEt O Cl O 2. H3O+ O OEt R O R O Ch. 18 - 40 7. Synthesis of Substituted Acetic Acids: The Malonic Ester Synthesis O EtO O OEt Diethyl malonate O EtO O OEt is the synthetic equivalent of: O O and OEt O Ch. 18 - 41 O R O EtO OH O OEt O R OH R' Ch. 18 - 42 Synthesis of monoalkylacetic acid O O O OEt EtO O EtO OEt OEt R H O O HO heat 1. NaOH (aq) OH 2. H3O+ O HO OEt R O OH O O R O EtO R H O X HO R HO R Ch. 18 - 43 Synthesis of dialkylacetic acid O O EtO O 1. EtONa OEt 2. RX O EtO OEt R 1. tBuOK, tBuOH 2. R'X O O HO OH R O 1. NaOH (aq) 2. H3O+ EtO R' OEt R R' O heat (- CO2) O R HO R' Ch. 18 - 44 Example 1 O EtO O O 1. EtONa, EtOH OEt 2. O EtO OEt Br 1. 50% KOH, reflux 2. dil. H2SO4, reflux O HO (Heptanoic acid) O (-CO2) HO O OH Ch. 18 - 45 Example 2 O O O 1. EtONa, EtOH OEt 2. MeI EtO O EtO OEt Me 1. tBuOK, tBuOH 2. Ph O HO Me O O 1. NaOH (aq) OH Ph 2. H3O+ Me O OEt Ph O 180oC (- CO2) EtO Br HO Ph Me Ch. 18 - 46 8. Further Reactions of Active Hydrogen Compounds Z Z' Active hydrogen compound (Z and Z' are electron withdrawing groups) Z, Z': O O R H O O S S R O O O NR2 OR O R S O N NO2 O OR or S O NR2 Ch. 18 - 47 Example O NC O 1. EtONa, EtOH OEt 2. NC Br O OEt 1. tBuOK, tBuOH NC OEt 2. Ph Br Ph Ch. 18 - 48 9. Synthesis of Enamines: Stork Enamine Reactions O C C H Aldehyde or ketone OH + HN R C R C R N R H 2o Amine R N C C R + H2O Enamine Ch. 18 - 49 2° amines most commonly used to prepare enamines O N H Pyrrolidine N H Piperidine N H Morpholine ● e.g. O N N H p-TsOH, H2O Ch. 18 - 50 N (a) (a) (b) +X N +R N R C-alkylated product +X O H product X R = H2C CH or Ph + N-alkylated heat (b) N R R H2O Ch. 18 - 51 Synthesis of b-diketones N O N H N O R Cl O R p-TsOH Cl (enamine) O N O R H2O O R Ch. 18 - 52 Synthesis of g-keto esters N O N H OEt Br O p-TsOH (enamine) N O OEt O OEt H2O O Ch. 18 - 53 Enamines can also be used in Michael additions N + CN EtOH N CN reflux O CN H2O Ch. 18 - 54 10. Summary of Enolate Chemistry 1. Formation of an Enolate O O + :B R R Resonancestabilized enolate H O H:B + R Ch. 18 - 55 2. Racemization R' R H O OH Ph or H3O R' R OH OH Ph or H3O R' H O R Ph Enol (achiral) Enantiomers Ch. 18 - 56 3. Halogenation of Aldehydes & Ketones O O R' R + X2 H acid or base R' R X Specific example: haloform reaction O O H Ph H H OH + 3 X2 H2O X Ph X X O CHX3 + Ph O Ch. 18 - 57 4. Halogenation of Carboxylic Acids: The HVZ Reaction O O R 1. X2, P OH 2. H2O R OH X Ch. 18 - 58 5. Direct Alkylation via Lithium Enolates O R O LDA, THF o H(R') -78 C R R'' X H(R') (formation of the kinetic enolate) O R H(R') R'' Specific example: O O Li LDA, THF O CH3I -78oC Ch. 18 - 59 6. Direct Alkylation of Esters O O LDA R OEt THF OEt R' O R R Br OEt R' Ch. 18 - 60 7. Acetoacetic Ester Synthesis O O 1. NaOEt OEt O OEt 1. OH, heat 2. H3O+ R 1. BuOK O O 2. R'Br OEt R O R R' R 3. heat, ( CO2) t OEt R O 2. RBr O O O R' 1. OH, heat 2. H3O+ 3. heat, ( CO2) Ch. 18 - 61 8. Malonic Ester Synthesis O O EtO 2. RBr OEt R HO O EtO R 2. R'Br R O EtO OEt R O R' R O 1. BuOK HO OEt 3. heat, ( CO2) t OEt O EtO 1. OH, heat 2. H3O+ O O O 1. NaOEt R' 1. OH, heat 2. H3O+ 3. heat, ( CO2) Ch. 18 - 62 9. Stork Enamine Reaction O R NR'2 R + R' NH 2 R Enamine O R R 1. R'' R Br 2. heat 3. H2O R'' Ch. 18 - 63 END OF CHAPTER 18 Ch. 18 - 64