easter lab
advertisement

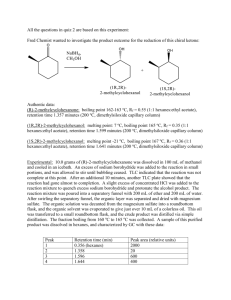
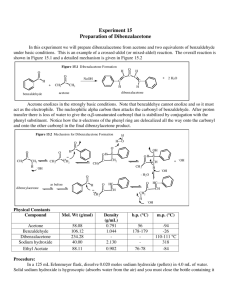
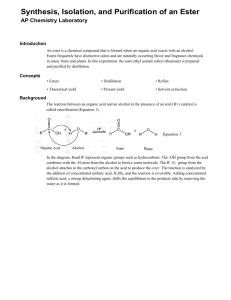
Preparation of Esters An ester is a chemical compound that is formed when an organic acid reacts with an alcohol. Esters frequently have distinctive odors, and are found in the flavorings of many fruits and plants_ The reaction between an organic acid and an alcohol is shown in Figure 1. Organic Acid Alcohol Ester Figure 1. The Reaction Between An Organic Acid and Alcohol to Form an Ester and Water In the diagram, Rand R' represent organic groups such as hydrocarbons. The -OH group from the acid combines with the -H from the alcohol producing water molecules. The R' -0- group from the alcohol then attaches to the carbon on the acid forming the ester. The reaetion is catalyzed by adding some concentrated sulfuric acid, H2S04, Concentrated sulfuric acid is a strong dehydrating agent, and helps the reaction by removing the water molecules as they are formed. If acetic acid and methanol are reacted, the reaction shown in Figure 2 occurs. The product is called methyl acetate. The systematic name for acetic acid is ethanoic acid, and the product is also known as methyl ethanoate. Figure 2. The Reaction between Acetic Acid (Ethanoic Acid) and Methanol In this experiment we will prepare small quantities of several esters. The esters will be identified by their distinctive odors. Then we will prepare a larger quantity of the ester ethyl acetate (ethyl ethanoate) and purify it by distillation. Chemicals Part 1: Various combinations of acids and alcohols will be used which may contain the following: Organic acids: Formic acid (methanoic acid) Acetic acid (ethanoic acid) Propionic acid (propanoic acid) Butyric acid (butanoic acid) Salicylic acid Anthranilic acid Organic alcohol: Methyl alcohol (methanol) Ethyl alcohol (ethanol) Propyl alcohol (n-Propanol) Isopropyl alcohol (isopropanol) Butyl alcohol (butanol) Isopentyl alcohol or isoamyl alcohol (isopentanol) Octyl alcohol (octanol) Sulfuric acid, concentrated (18 M) Baking soda, NaHC03, to neutralize acid spills Part 2: Acetic acid, concentrated, 17.4 M (glacial) Ethanol (ethyl alcohol, denatured alcohol) Sulfuric acid, concentrated (18 M) Sodium carbonate, N~C03·lOH20, solid Baking soda, NaHC03, to neutralize acid spills Equipment Part 1: Test tubes, 13- x 100-mm Beaker, 250-mL Beaker, 400-mL Hot plate or Bunsen burner, ring and wire gauze Part 2: Boiling stone Erlenmeyer flask, 125-mL Condenser with cork fittings Thermometer Distilling flask Clamps Hot plate, or Bunsen burner, ring and wire gauze Beaker, 400-mL for water bath Test tube, 18 x I50-mm and cork stopper Capillary Beaker, lOO-mL to collect distillate dropper Separatory funnel or test tube, 15- x 125-mm, and stopper and return to the reaction vessel. Slowly run cold water through the condenser, in at the bottom and out at the top. Heat the flask in a hot water bath. Raise the temperature of the hot water until the mixture in the Erlenmeyer flask is gently boiling, and continue heating for about 15 minutes. Cool the mixture. 2. Distillation of ethyl acetate. Pour the mixture (including the boiling stone) into a distilling flask and connect the condenser to the side arm of the flask. Insert a thermometer in an aluminum foil-covered cork in the top of the flask with the 2. 3. thermometer bulb even with the side arm of the condenser. Heat the bottom of the distilling flask in a hot water bath until no more distillate is coming over. Record the temperature at which the distillation begins and the temperatures during and at the end of the distillation. Look up the boiling point of ethy 1 acetate and compare to the distillation temperature. SET UP A DISTILLATION PROCESS 3. Separation of the ethyl acetate from alcohol. During the distillation some of the unreacted alcohol will distill along with the ethyl acetate. Ethanol is very soluble in a saturated solution of sodium carbonate, while the ethyl acetate is only slightly soluble. Do not distill to dryness. Use extreme caution when distilling mixtures containing flammable liquids. Do not distill liquids with boiling points below SO°C. Use only cold water in the condenser. If available, use an electric heating mantle instead of a laboratory burner. Prepare a saturated solution of sodium carbonate in distilled water by combining 1.5 g N~C03·lOH20 with 5 mL distilled water in a 15 x 2s-mm test tube. Stopper with a cork, shake well and then allow any undissolved solid to settle. Pour the clear solution into a separatory funnel, or if none is available, into a second 18- x Is0-mm test tube. Add the distillate, stopper and shake for a minute. If using a separatory funnel, turn it upside down and open the stopcock occasionally to vent the system. If using a test tube, remove the stopper with caution-some pressure may have built up. Separate the two layers. A capillary dropper may be helpful in the separation if you are using a test tube. Measure the mass of the ethyl acetate produced. Pour a little of the ethyl acetate into 200 mL of water and cautiously note its odor. Disposal The solutions used to prepare the esters can be safely washed down the sink with a large amount of water according to Flinn Suggested Disposal Method #26b. The ethyl acetate can be saved and used as a solvent, or can be evaporated in the fume hood according to Flinn Suggested Disposal Method #18a. See the appendix. Discussion In your laboratory report include all of your observations, and answer the following questions: 1. The density of ethanol is 0.79 g/mL. The density of acetic acid is 1.05 g/mL. Assuming that each substance was a pure substance, calculate the moles of each reactant used in part 2. Determine the limiting reactant, and calculate the theoretical yield of ethyl acetate. Use the actual yield to determine the percent yield of product. 2. Why was sulfuric acid added to the mixture of acid and alcohol?