Quiz 5
advertisement

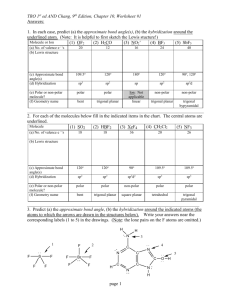
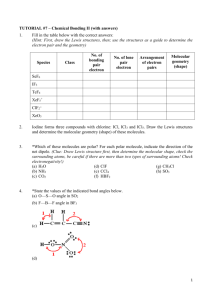
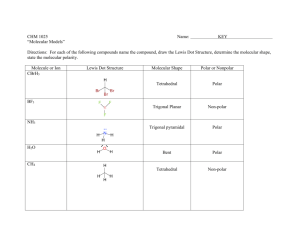
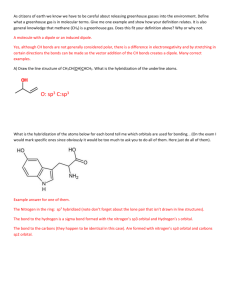
Quiz 4 1) Chemistry 172 (Winter 08-09) Name _____________________ Predict whether a sigma (s), pi (p), or no bond (n) forms from the following orbitals coming together on the x axis. a) px and py __n___ b) px and px __s___ c) sp3 and s __s___ d) sp3 and px __s___ e) sp2 and sp __s___ e) sp3 and py __n___ For 2-4, refer to the structure below: 2) There are ___13______ sigma bonds and _____3_____ pi bonds. 3) Identify the expected hybridization of each atom. A (carbon) __sp____ B (carbon) ___sp2____ C (nitrogen) ___sp3____ 4) Provide the value of each indicated angle. 1 (C-C-C) _180__ 5) 6) 2 (C-C-C) _120__ 3 (C-N-H) _109.3___ As the number of bonds between two carbons increases, which one of the following decreases? a) number of electrons between the carbon atoms b) bond energy c) bond length d) formal charge Draw the number and relative energy of 2s and 2p orbitals of an atom before hybridization and the number and relative energy levels of the orbitals that are formed in the atom after sp hybridization before hybridization 2p ____ _____ _____ after hybridization 2p ____ _____ sp ____ _____ 2s ____ 7) Provide a rationale as to why the 1s orbitals are not included in the formation of hybrid orbitals. Valence electrons are not in the 1 s orbitals and not involved in bonding. They are smaller than the 2s and 2p orbitals and may not combine as well 8) Identify the type of hybridization in the oxygen and the carbons below. Describe what orbitals are employed to form each of the bonds in this molecule. O is sp3 hybridized and C is sp2 hybridized The sp3 orbitals of O form a σ bond with the 1s of H and with the sp2 of C The sp2 orbitals of the right C form σ bonds with 1s of H, sp3 of O, and sp2 of the other C. The sp2 orbitals of the left C form σ bonds with 1s of H, sp2 of the other C The unhybridized p of each C forms a π bond. 9) For each molecule below provide the requested information in the appropriate blank. A correct Lewis dot structure is required to answer these correctly. a) SeO3 b) PF3 c) SF4 d) SiCl4 e) HCN f) PCl5 g) IF5 ______sp2______ hybridization __non-polar______ polar or non-polar trigonal planar_ electron arrangement trigonal planar molecular shape _____sp3_______ hybridization __polar___ polar or non-polar tetrahedral____ electron arrangement trigonal pyramidal molecular shape ____dsp3______ hybridization polar________ polar or non-polar trigonal bipyramidal electron arrangement seesaw_______ molecular shape ___sp3_________ hybridization _ nonpolar_ polar or non-polar _tetrahedral___ electron arrangement _tetrahedral molecular shape _sp__________ hybridization ___polar______ polar or non-polar _linear_______ electron arrangement __linear______ molecular shape _dsp3_________ hybridization _nonpolar__ polar or non-polar trigonal bipyramidal electron arrangement trigonal bipyramidal molecular shape ___d2sp3_______ hybridization _polar_______. polar or non-polar octahedral_ electron arrangement _square pyramidal_ molecular shape a) SeO3 b) PF3 c) SF4 d) SiCl4 e) HCN f) PCl5 g) IF5 ______________ hybridization ______________ polar or non-polar ______________ electron arrangement ______________ molecular shape ______________ hybridization ______________ polar or non-polar ______________ electron arrangement ______________ molecular shape ______________ hybridization ______________ polar or non-polar ______________ electron arrangement ______________ molecular shape ______________ hybridization ______________ polar or non-polar ______________ electron arrangement ______________ molecular shape ______________ hybridization ______________ polar or non-polar ______________ electron arrangement ______________ molecular shape ______________ hybridization ______________ polar or non-polar ______________ electron arrangement ______________ molecular shape ______________ hybridization ______________. polar or non-polar ______________ electron arrangement ______________ molecular shape