Color and Bonding in Transition Metal Complexes
advertisement

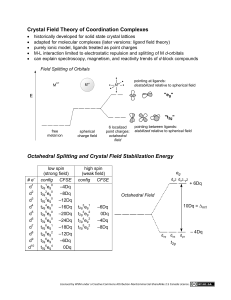
Color and Bonding in Transition Metal Complexes Chemistry 123 Dr. Patrick Woodward Supplemental Lecture 3 Intra-atomic (localized) excitations – – Transition metal ions, complex ions & compounds (d-orbitals) Lanthanide ions and compounds (f-orbitals) [Ni(NH3)6]2+ NiSO4 Cu3(CO3)2(OH)2 CuSO4 Malachite In these complexes the color comes from absorption of light that leads to excition of an electron from an occupied d-orbital to an empty or ½-filled d-orbital. The energy separation between d-orbitals depends upon the interaction between the dorbitals and the ligands. There are two ways to rationalize the energy separation Crystal Field Theory & Ligand Field Theory. 1 Energy Crystal Field Splitting (Octahedron) dz2 & dx2-y2 orbitals (e (eg) •Point directly at the ligands •Stronger (repulsive) interaction with the ligands dxy, dyz & dxz orbitals (t2g) dz2 dx2-y2 dyz dxz •Point between the ligands •Weaker (repulsive) interaction with the ligands dxy Electrons in d-orbitals are repelled from the electrons in the ligands, based on electrostatic interactions. This causes two of the d-orbitals (dz2 & dx2-y2) to be at higher energy than the other three (dxy, dyz, dxz) Ligand Field Splitting (Octahedron) dz2 & dx2-y2 orbitals (e (eg) •Point directly at the ligands •Sigma antibonding interaction with the ligands dz2 σ* dx2-y2 σ* dxy, dyz & dxz orbitals (t2g) •Point between the ligands •Pi antibonding interaction with the ligands dxy π* dyz π* dxz π* Ligand field theory is based on covalent interactions between the metal and the surrounding ligands, we can use MO theory to understand it. The splitting of orbitals into a lower energy t2g set of orbitals (non bonding, or piantibonding) & and a higher energy eg set of orbitals (sigma antibonding). 2 d-orbital Splitting (Octahedron) dz2 dx2-y2 dz2 & dx2-y2 orbitals (e (eg) eg •Point directly at the ligands •Sigma antibonding interaction with the ligands Δ = Crystal Field Splitting Energy dxy, dyz & dxz orbitals (t2g) •Point between the ligands •Pi antibonding interaction with the ligands t2g dxy dyz dxz Cr3+ 5 dd-orbitals on Cr (Cr3+ = d3 ion) 3 electrons in the d-orbitals [Cr(NH3)6]3+ Octahedron :NH3 : N H HH 6 Ligand Orbitals Nitrogen lone pairs (all containing 2 e-) Only sigma interactions are allowed 3 [Cr(NH3)6]3+ Antibonding (σ*) Metal-Ligand MO’s eg orbitals Δ = Crystal Field Splitting Energy t2g orbitals Energy Metal (Cr) d-orbitals Nonbonding Metal d MO’s Nonbonding Ligand MO’s Ligand (N) lone-pair orbitals Δ ~ 3.0 eV (~410 nm) Absorption = Violet Color = Yellow Bonding (σ) Metal-Ligand MO’s eg eg Δ Δ t2g t2g Cl– Small Δ Spectrochemical Series < F- < H2O < NH3 < NO2- < CN- Weak Field Ligand Weak M-L interaction Large Δ Strong Field Ligand Strong M-L interaction 4 High spin & low spin states Large Δ Small Δ Low Spin Configuration High Spin Configuration The t2g set of d-orbitals are completely filled before electrons fill the eg orbitals All five dorbitals are filled before pairing up 2 electrons in one orbital Diamagnetism – All electrons are paired up, which leads to equal numbers of spin up and spin down electrons (i.e. [Co(CN)6]3-) Paramagnetism – Unpaired electrons, which leads to unequal numbers of spin up and spin down electrons ((i.e. [CoF6]3-) Cr3+ Gemstones Corundum - Al2O3 Beryl - Be3Al2Si6O18 Ruby Al2O3:Cr3+ In both gemstones Cr3+ substitutes for Al3+, which is surrounded by 6 oxygen ions in an octahedron. The color comes from a d-to-d excitation on the Cr3+ center. Emerald Be2Al2Si6O18:Cr3+ 5 Measurements show that the crystal field splitting, Δ, of the Cr3+ ion in ruby and emerald are: 1. Ruby = 2.3 eV (540 nm) & Emerald = 1.9 (650 nm) 2. Ruby = 1.9 eV (650 nm) & Emerald = 2.3 (540 nm) 50% 10 50% Ruby = 2.3 eV (540 nm) & Emerald = 1.9 (650 nm) Ruby = 1.9 eV (650 nm) & Emerald = 2.3 (540 nm) Do you expect the Cr-O distances to be shorter in ruby (Δ = 2.3 eV) or emerald (Δ = 1.9 eV) 1. Shorter in Ruby 2. Shorter in Emerald 50% 50% 10 Shorter in Ruby Shorter in Emerald 6 Ligand Field Splitting (Tetrahedron) dxy, dyz & dxz orbitals (t2) •Stronger antibonding interaction with the ligands •Higher energy dxy dyz dxz dz2 & dx2-y2 orbitals (e) •Weaker antibonding interaction with the ligands •Lower energy dz2 dx2-y2 The crystal field splitting, Δ, for a tetrahedron is considerably smaller than for an octahedron Ligand Field Splitting (Tetrahedron) dxy, dyz & dxz orbitals (t2) dxy dyz dxz •Stronger antibonding interaction with the ligands •Higher energy t2 Δ = Crystal Field Splitting Energy dz2 & dx2-y2 orbitals (e) •Weaker antibonding interaction with the ligands •Lower energy e dz2 dx2-y2 The crystal field splitting, Δ, for a tetrahedron is considerably smaller than for an octahedron 7 5 dd-orbitals on Cr (Cr6+ = d0 ion) 0 electrons in the dd-orbitals Cr3+ O 4 Ligand Orbitals Oxygen lone pairs (all containing 2 e-) CrO42- Tetrahedron [CrO4]2- t2 orbitals (antibonding) e orbitals (antibonding) CT Energy Metal (Cr) d-orbitals Nonbonding Oxygen 2p MO’s e orbitals (bonding) t2 orbitals (bonding) PbCrO4 12 Oxygen 2p orbitals (4 oxygens x 3 p orbitals) CT ~ 3.3 eV (~375 nm) Absorption = Violet Color = Yellow 8 Charge Transfer in Sapphire • The deep blue color the gemstone sapphire is also based on impurity doping into Al2O3. The color arises from the following charge transfer excitation: Fe2+ + Ti4+ → Fe3+ + Ti3+ (λmax ~ 2.2 eV, 570 nm) • The transition is facilitated by the geometry of the corundum structure where the two ions share an octahedral face, which allows for favorable overlap of the dz2 orbitals. Fe2+ Ti4+ • Unlike the d-d transition in Ruby, the chargetransfer excitation in sapphire is fully allowed. Therefore, the color in sapphire requires only ~ 0.01% impurities, while ~ 1% impurity level is needed in ruby. 9