Lecture 04 Chem 3
advertisement

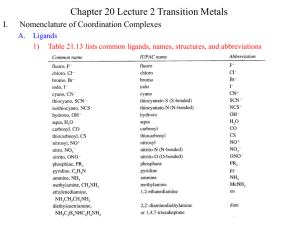
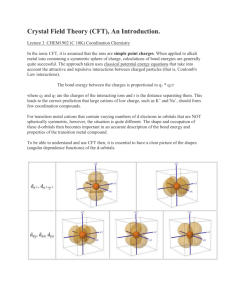
Atom Config Electrons 1s1 1s2 1 2 1s22s1 1s22s2 1s22s22p1 1s22s22p2 1s22s22p3 1s22s22p4 1s22s22p5 1s22s22p6 3 4 5 6 7 8 9 10 n=1 H He n=2 Li Be B C N O F Ne n=3 Na Mg Al Si P S Cl Ar 1s22s22p63s1 1s22s22p63s2 1s22s22p63s23p1 1s22s22p63s23p2 1s22s22p63s23p3 1s22s22p63s23p4 1s22s22p63s23p5 1s22s22p63s23p6 11 12 13 14 15 16 17 18 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn 1s22s22p63s23p63d04s1 1s22s22p63s23p63d04s2 1s22s22p63s23p63d14s2 1s22s22p63s23p63d24s2 1s22s22p63s23p63d34s2 1s22s22p63s23p63d54s1 1s22s22p63s23p63d54s2 1s22s22p63s23p63d64s2 1s22s22p63s23p63d74s2 1s22s22p63s23p63d84s2 1s22s22p63s23p63d104s1 1s22s22p63s23p63d104s2 19 20 21 22 23 24 25 26 27 28 29 30 3d metals (8 First transition series metals constitute the bulk of essential microminerals to life) Definition: What is a transition element? An element in the periodic table characterized by having partially filled d orbitals, created by having the adjoining s orbitals filled before the d. Properties: The 3d orbitals are split by ligands resulting in orbitals with higher and lower energy states that supersede the 5 degenerate orbitals. Characterized by Multi-valence states Importance: Resulting complexes take on specific geometrical shapes that relate to binding, color formation, and functionality Important Definitions Ligand: (Lat: that which ties) A ligand is a charged or neutral molecule that binds to a metal through either coordinate covalent or ionic bonds. Water is a neutral ligand, CN is a charged ligand. Chelator: (Lat. Claw) A chelator is an organic compound that is capable of wrapping around a metal in multiple bonds thus competing with other molecules (e.g., proteins, nucleic acids) for the metal. Multidentate: ( Lat: dentate, teeth) Referring to a molecule that has multiple binding groups within the same chain capable of forming multiple bonds with the metal ion, e.g., bidentate (2) tridentate (3) etc. Orbital Splitting: A process by which d orbitals are split into high and low energy levels in response to the binding of a ligand. Coordination Number: Referring to the number of ligands that attach Coordinate covalent: A type of bond created when a ligand provides the pair of bonding electrons (Lewis base) to share with the metal. Multi-dentate Ligands O Oxalate O CH2-CH2 CC NH2 H 2N O O Co3+ Cu2+ Ethylene diamine O O O C-C OOC OOC CH2-CH2 .. N N .. COO COO Ethylenediamine tetraacetic acid (EDTA) O 3d orbitals Z Z Z Z X Y Y X dxy Z Y X dxz dyz X dX2-Y2 Y X Y dZ2 Octahedral Complex 3 of most common complexes with metal ions are: Octahedral (most common) An 8 sided figure featuring 6 ligands, 4 in one plane and two above and below the plane. Square planar A 4 sided figure with 4 ligands all in the same plane Tetrahedral 4 ligands vectorially positioned to have minimum interaction Transition metals that form octahedral complexes Fe Zn Ni Co Cr Mn Transition metals that form tetrahedral complexes Zn Cu Co Transition metals that form square planar and 5-coordination complexes Cu Zn Cu Orbital splitting Insights into the properties of ligands Take Home: By altering the energy state of electrons in a metal ion, ligands are capable of determining valence, reactivity, and even the color of the complex 3d Orbitals dx2-y2 dz2 Octahedral Iron Fe forms an octahedral (8 sided figure, six ligands) complex by having its 5, 3d orbitals split into two 2 new orbitals, eg and t2g. eg Before splitting x2-y2 z2 o Energy difference xy xz yz x2-y2 z2 t2g xy xz yz After splitting Ti = [Ar]4s23d2 x2-y2 Ti(II) = [Ar]3d2 Ti(III) = [Ar]3d1 Ti2+ Ti3+ hv z2 x2-y2 z2 Ti(III) One 3d xy xz Ground state t12g L L L Ti L L L yz xy xz yz Excited state e1g Feo [Ar]4s23d6 Ionizes (loses 4s2 electrons to form Fe2+) CN- as a ligand Fe2+ [Ar]3d6 (water as a ligand) [Fe(CN)6]4- x2-y2 xy Fe(II) z2 xz t62g Low Spin (Highly energetic) Diamagnetic [Fe(H2O)6]2+ yz x2-y2 xy z2 xz yz t42ge2g High Spin (Low energetic) Paramagnetic V [Ar]4s23d3 No low spin possible V(II) Cr [Ar]4s13d5 Cr(II) Mn [Ar]4s23d5 Mn(II) High Spin Low Spin Fe [Ar]4s23d6 Fe(II) Co [Ar]4s23d7 Co(II) Ni [Ar]4s23d8 Ni(II) No low spin possible Cu [Ar]4s13d10 No low spin possible Cu(I) Cu [Ar]4s13d9 Cu(II) Zn [Ar]4s23d10 Zn(II) No low spin possible No low spin possible Class Exercise: Draw the electronic configuration of octahedral [Zn(H2O)6]2+ and predict the color. Zn is [Ar]4s23d10 Solution Upon ionization, Zn loses its 2, 4s electrons and becomes 3d10 x2-y2 xy z2 xz All orbitals are filled, no color is possible yz Common Ligands Ligand Name FClBrICNNCSSCNOHO2ONOCO H 2O NH3 Fluoride Chloride Bromide Iodide Cyanide Isothiocyanate Thiocyanate Hydroxide Oxide Nitrite Carbon monoxide Water Ammonia Name as ligand Fluoro Chloro Bromo Iodo Cyano Isothiocyanato Thiocyanato Hydroxo Oxo Nitro Carbonyl Aqua Ammine Underline indicates atom bonded to metal Ligand Strength and Numbers as a determinant Rule: Ligands differ in the strength of their orbital splitting. The following has been determined experimentally Cl < F- < H2O < NH3 < NO2- < CN- < CO Rule: Low spin complexes are created by ligands with strong orbital splitting properties Rule: Octahedral complexes that have 3, 4, 5, or 6 electrons in the t2g orbital tend to be very stable (inert). All others are labile. Biological Relevance Myoglobin O=O Heme group Spherical90% -helix Interfere O2 binding to Heme His E7 C O A linear carbon monoxide can bind with less interference O2 binds above the ring plane Histidine binds below the plane of the ring Only Fe(II) will bind O2 Histidine F8 Ferrous (Fe(II) COLOR garnet aquamarine ruby amethyst topaz kyanite Red Blood vs Blue Blood O2 binding to the heme ring of hemoglobin is coordinated to iron (II). When O2 is bound to one of the coordinates, Fe(II) is in a low spin (high energy) state and the light emitted is a red. Without O2 the iron binds water resulting in high spin (low energy) and takes on a bluish color. red Hmb 4O2 red (low spin) Arterial blood blue Hmb + 4O2 blue (high spin) Venous blood