Crystal Field Theory of Coordination Complexes Octahedral Splitting
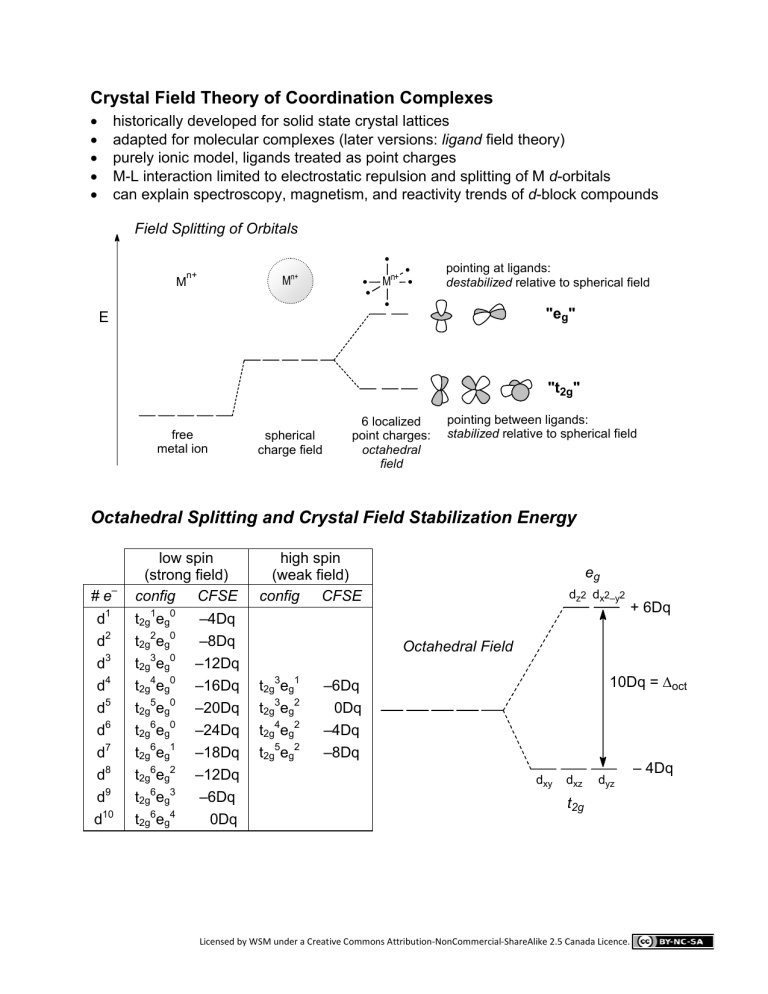
Crystal Field Theory of Coordination Complexes
•
historically solid state crystal lattices
•
adapted for molecular complexes (later versions:
ligand
field theory)
•
purely ionic model, ligands treated as point charges
•
M-L interaction limited to electrostatic repulsion and splitting of M
d
-orbitals
•
can explain spectroscopy, magnetism, and reactivity trends of
d
-block compounds
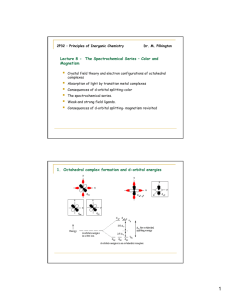
Field Splitting of Orbitals
E
M n+
•
•
•
M n+
•
•
• pointing at ligands: destabilized relative to spherical field
"e g
" free metal ion spherical charge field
"t
2g
"
6 localized point charges: octahedral field pointing between ligands: stabilized relative to spherical field
Octahedral Splitting and Crystal Field Stabilization Energy
spin high spin
(strong field) (weak field)
# e
–
config CFSE config CFSE
d
1 t
2g
1 e g
0
–4Dq d
2 t
2g
2 e g
0
–8Dq d
3 t
2g
3 e g
0
–12Dq d
4 t
2g
4 e g
0
–16Dq t
2g
3 e g
1
–6Dq d
5 t
2g
5 e g
0
–20Dq t
2g
3 e g
2
0Dq d
6 t
2g
6 e g
0
–24Dq t
2g
4 e g
2
–4Dq d
7 t
2g
6 e g
1
–18Dq t
2g
5 e g
2
–8Dq d
8 t
2g
6 e g
2
–12Dq d
9 t
2g
6 e g
3
–6Dq d
10 t
2g
6 e g
4
0Dq
Octahedral Field
d xy d z2
e
g d x2–y2
+ 6Dq d xz
t
2g d yz
10Dq =
Δ oct
– 4Dq
Licensed by WSM under a Creative Commons Attribution ‐ NonCommercial ‐ ShareAlike 2.5
Canada Licence.
Tetrahedral Splitting
An alternate view of a tetrahedron: a cube with half the corners missing
Three orbitals point at ligands
•
• z y
•
•
d xz
d yz
d xy
Two orbitals point between ligands
• •
Tetrahedral Field x d xy
" t
2
" d xz d yz
+ 1.78Dq
• •
• •
•
• •
• y x d z 2 • • d x
2
–y
2 d z2 d x2–y2
" e "
∆ tet
splitting is inverse of ∆ oct
: two below three rather than three below two
∆ tet
splitting ≈ ½ ∆ oct
: tetrahedral compounds are almost always HS
4.45Dq = ∆ tet
– 2.67Dq
Crystal Field Splitting for Common Geometries (Dq units) d x2–y2
+12.28
d z2
+10.28
d z2 d z2 d x2–y2
+6.00
+7.07
E d xy d xz d yz
+1.78
d xy
+2.28
0 Dq d xy d xz d yz
–4.00
octahedral d z2 d x2–y2
–2.67
tetrahedral d z2 d xz d yz square planar
–4.28
–5.14
d xz d yz
+1.14
d xy d xz yz
–0.82
d x2–y2
–2.72
d d xy d x2–y2
–6.28
linear trigonal bipyramidal
Licensed by WSM under a Creative Commons Attribution-NonCommercial-ShareAlike 2.5 Canada Licence.
Factors that Affect
∆
1) Number of ligands and geometry (see previous)
2) Oxidation state: ∆↑ as n+ ↑ ( Note : n is oxidation state, not principal QN)
∆ o
(cm
–1
)
∆ o
(cm
–1
) ML n
[Cr(H
2
O)
6
]
2+
[Cr(H
2
O)
6
]
3+
14100
17400
ML n
[Fe(H
2
O)
6
]
2+
[Fe(H
2
O)
6
]
3+
9400
13700
3) Period: ∆↑ down a column
ML n
∆ o
(cm
–1
)
[Co(NH
3
)
6
]
3+
22900
[Rh(NH
3
)
6
]
3+
24000
[Ir(NH
3
)
6
]
3+
41200
4) Ligands: Spectrochemical Series
ML n
[CrF
6
]
3–
∆ o
(cm
–1
)
15000
17500
ML n
[CoF
6
]
3–
∆ o
(cm
–1
) ML n
13100 [FeCl
6
]
3–
∆ o
(cm
–1
)
11000
[Co(H
2
O)
6
]
3+
18200 [Fe(H
2
O)
6
]
3+
13700 [Cr(H
2
O)
6
]
3+
[Cr(NH
3
)
6
]
3+
[Cr(CN)
6
]
3–
21500
26700
[Co(NH
3
)
6
]
3+
22900 [Fe(NH
3
)
6
[Co(CN)
6
]
3+
33500 [Fe(CN)
6
]
]
3+
17500
3–
32800
Spectrochemical Series: Ligand Effect on ∆
I – < Br – < Cl – < ONO – < F – < OH – < H
2
O < MeCN < py < NH
3
< en < bpy < phen < NO
2
– < PR
3
< CN – < CO
←→ weaker field, smaller splitting stronger field, larger splitting
Some rules of thumb about the magnitude of ∆ :
• Tetrahedral complexes tend to be high spin
• Octahedral complexes will be high spin only if
• first row transition metal (3d), AND
• either weak field ligand or low oxidation state
An aside: cm
–1
= wavenumbers, a unit of energy favoured by certain breeds of spectroscopist
ν = = so E = h
ν
= = hc ν 1000 cm
–1
≈ 12 kJ/mol
Licensed by WSM under a Creative Commons Attribution-NonCommercial-ShareAlike 2.5 Canada Licence.