Advanced Inorganic Chemistry CHEM4305 Spring `04
advertisement
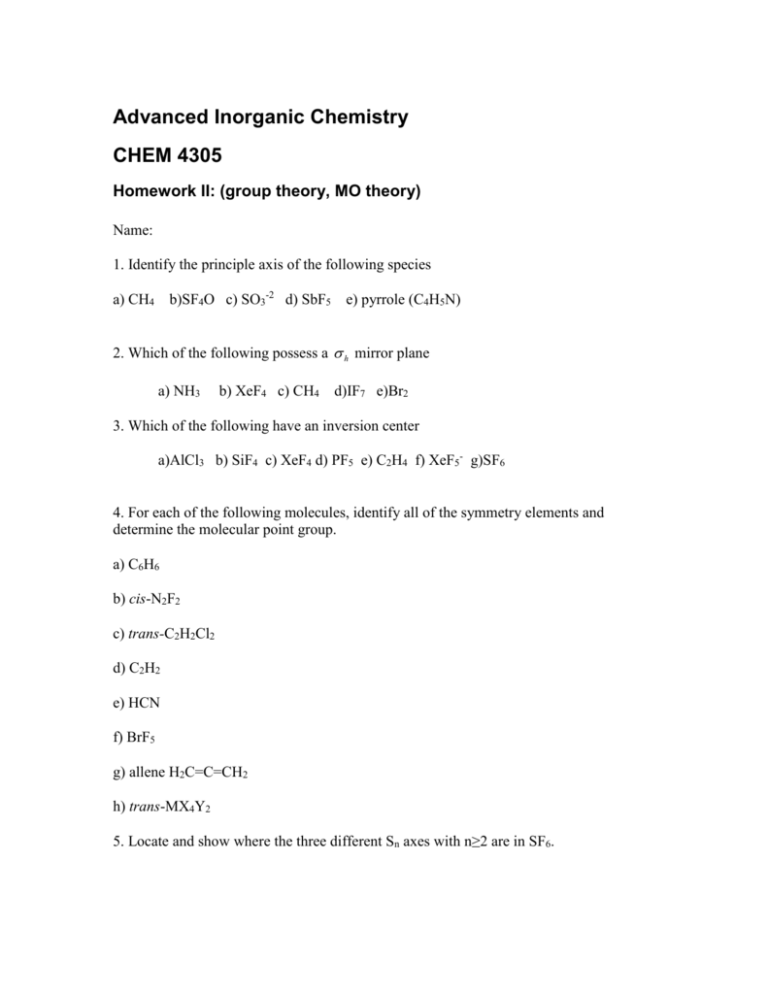
Advanced Inorganic Chemistry CHEM 4305 Homework II: (group theory, MO theory) Name: 1. Identify the principle axis of the following species b)SF4O c) SO3-2 d) SbF5 a) CH4 e) pyrrole (C4H5N) 2. Which of the following possess a h mirror plane a) NH3 b) XeF4 c) CH4 d)IF7 e)Br2 3. Which of the following have an inversion center a)AlCl3 b) SiF4 c) XeF4 d) PF5 e) C2H4 f) XeF5- g)SF6 4. For each of the following molecules, identify all of the symmetry elements and determine the molecular point group. a) C6H6 b) cis-N2F2 c) trans-C2H2Cl2 d) C2H2 e) HCN f) BrF5 g) allene H2C=C=CH2 h) trans-MX4Y2 5. Locate and show where the three different Sn axes with n≥2 are in SF6. 6. What are the symmetries of the normal vibrational modes for following molecules? a) NF3 b) OF2 7. Consider the following ABn molecules and determine the symmetries and degeneracies of the s,p, and d orbitals of A in each. a) AB8 (cube) b) AB4 (square planar) c) AB3 (trig pyramidal) d) AB3 (trig. Planar) e) AB3 (T shaped) f) AB4 (rectangular plane) 8. For each of the following molecules, determine what atomic orbitals on the central atom are allowed by symmetry to be used in the construction of sigma hybrid orbitals. a) NH3 b) BF3 c) SF6 d) PF5 (trig. Bipyramidal) 9. For the following molecules, determine the number of IR active C-O stretching vibrations: CO OC Cl Cl OC Ru OC a) CO Ru Cl CO OC b) CO Cl 10. Using the D2d character table, a) Determine the order of the group b) Reduce the following representations to their component irreducible representations: D2d │E 2S4 C2 2C2’ 2σd Γ1 │6 0 2 2 2 Γ2 │6 4 6 2 0 11. Draw the MO energy level diagram for BeCl2 (assume linear conformation) and indicate which orbitals are involved in bonding and which are non-bonding. 12. For a square planar metal-ligand coordination complex, determine the types of hybridization possible (sigma only) for a metal surrounded by four ligands. Use the appropriate character table. 13. The following diagram shows the hybridization schemes for three sp2 orbitals. Show that the wavefunctions are normalized and correspond to the structures given below: 1 2s 3 1 2s 3 1 2s 3 sp 2 sp sp 2 2 2 2p 3 x 1 1 2 px 2 p y 6 2 1 1 2 px 2 p y 6 2 14. The table listed gives the results of a Fenske-Hall SCF (self-consistent field) ab initio calculation for water using an orbital basis set of the oxygen orbitals and the LGO’s from the fragment H—H. The axis set is defined in the diagram below. a) Use the computational results to construct pictorial representations of the MO’s of H2O and b) confirm that the diagram is consistent with the computational results. c) How does the MO theory account for the lone pair electrons in H2O? Percentage character of MO’s with the sign of eigenvector in () A O or LGO O 2s O 2px O 2py O 2pz H--H 1 H--H 2 Ψ1 Ψ2 Ψ3 Ψ4 Ψ5 Ψ6 71(+) 0 0 0 29(+) 0 0 0 59(+) 7(-) 0 0 85(-) 8(+) 0 0 100(+) 0 0 0 0 0 0 41(-) 0 0 59(-) 22(-) 0 0 15(+) 63(+) 0 0 41(-) 15. The I-I bond distance in the gas phase I2 is 267 pm and in [I3]+ is 268 pm and in [I3]is 290 pm. a) Draw Lewis structures for these species. Do these structures account for the bond length variations indicated? b) Use MO theory to describe the bonding and deduce the I-I bond order in each case. Are these results consistent with the structural data? 16 Sketch a reasonable MO for SO. Would you expect SO to be diamagnetic or paramagnetic? 17.Sketch an MO diagram for the NO- ion and write the MO configuration. What is the bond order, the number of unpaired electrons, and where would any unpaired electrons reside (on the N or the O?) 18. Consider the MO’s for XH2. Which MO is stabilized (energy is lowered) by bending. Which is destabilized? Use your conclusions to explain (briefly) why BeH2 is linear, and H2O is bent