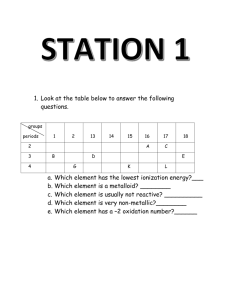
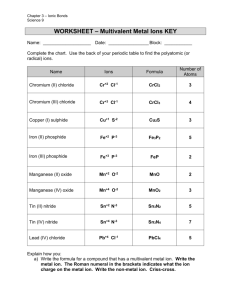
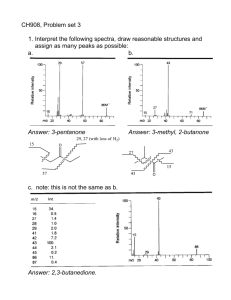
Transition elements
advertisement

Transition metals Transition Metals Coloured Compounds Diagram 1 Absorption of light energy Diagram 2 Example: a blue coloured compound arises because: Experiment – Chromium Experiment – Manganese Experiment – Manganese ctd Experiment – Iron Experiment – Copper Experiment – Vanadium (DEMO) Transition metal colours Start Transition Metals from Sc to Zn electrons are filling the 3d orbitals (4s filled already except Cr and Cu) A transition element has AT LEAST ONE ION with a PARTIALLY FILLED d-SHELL (so Sc, Sc3+ and Zn aren’t – no ion with partially filled d-shell) Start Coloured Compounds All 5 d-orbitals in an isolated transition element have the same energy. When ligands bond to the central ion, the d-orbitals move up to two different energy levels. The gap between the energy levels depends upon – the ligand, the coordination number and the transition metal ion. Start Diagram 1 Ligands split the 3d energy level 3d orbitals in a Cu2+ ion Energy gap, DE Start Absorption of light energy An electron can be promoted from the lower 3d energy level by absorbing energy EXACTLY equal to the energy gap. This energy is provided by radiation from the visible and UV regions of the spectrum. Start Diagram 2 Absorption of light energy Cu(H2O)62+ in an excited state Promotion of electron to higher energy level Start Example: a blue coloured compound arises because: absorption of light energy in the red, yellow, and green regions of the spectrum. This absorbed radiation provides the energy for an electron to be excited to a higher energy level reflection of blue light only The colour of a transition metal complex ion results from the transfer of an electron between the orbitals of an unfilled d subshell Start Transition metal colours Zn(II) (ZnSO4) colourless Cu(I) (Cu2O) brick red, Cu(II) blue (with some black compounds), CuI white. Mn(VII) (MnO4-) purple, Mn (VI) (MnO42-) green, Mn(IV) (MnO2) brown, Mn(II) (Mn2+) colourless/pale pink V(V) (VO2+)orange, V(IV) (VO2+) blue, V(III) (V3+) (green) V(II) (V2+) violet Fe(II) pale green, Fe(III) orange