ED MANAGEMENT OF DKA IN CHILDREN
advertisement

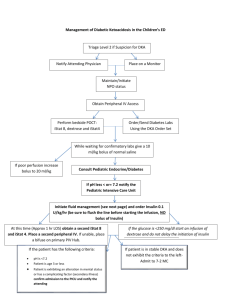
ED Management of DKA In Infants and Children DKA?1 No Yes Obtain on all patients: rapid blood glucose, ABG, UA serum glucose/lytes/Ca/Mg/PO4/BUN/creatinine Hgb A1C, 12 lead ECG Initiate fluid therapy3 Initiate insulin therapy4 Monitor closely for & treat cerebral edema5 Monitor dextrose hourly6 Pediatrics/Pediatric Endocrinology Consult Admit 1 Guideline for ED Management of DKA in Children Management considerations2 Notes: 1 DKA=blood glucose > 250 mg/dL + academia (pH < 7.30 or serum TCO2 < 15 mEq/L) + ketonuria 2 For pH > 7.3 & TCO2 > 15 mEq/L: a. hydrate (consider NS 20 mL/kg IV) a. give regular insulin (consider correction factor of 1 unit regular for every 25-50 mg/dL over 150 mg/dL) c. send home if: alert; able to drink & retain oral fluids; constant supervision is available; home blood glucose monitoring can be performed; all necessary supplies are available at home; suitable physician follow-up has been arranged; not new-onset d. suggested home management: (1) calculate 1 U/kg as total daily dose (TDD) of insulin (2) give 35-55% of TDD as Lantus qHS (3) obtain rapid blood glucose qAC (4) give 1 U regular insulin for every 10-15 carbs per meal/snack (5) give 1 U regular insulin for every 25-50 mg/dL over 150 mg/dL per meal/snack 3 Fluid therapy: a. HOUR 1: give NS 10 mL/kg IV bolus; additional fluid should be given only after careful patient assessment for evidence of dehydration; DO NOT GIVE > 20 mL/kg NS b. HOUR 2: give D10W ½ NS with 20 mEq KCl/L and 20 mM KPhos/L (IVF #1) + ½ NS with 20 mEq KCl/L and 20 mM Kphos/L (IVF #2) at total rate (cc/hr) calculated as 1-1 ½ maintenance (maintenance=4 cc/hr for 1st 10 kg + 2 cc/hr for 2nd 10 kg + 1 cc/hr for each additional kg) 4 Insulin: start IV infusion 0.1 U/kg/hr 5 For signs of cerebral edema (deterioration in mental status, change in neuro exam, signs of increased ICP i.e. increased MAP/bradycardia/change in respiratory pattern): obtain emergent head CT; initiate mannitol 1 g/kg IV; RSI; ventilate to maintain pCO2 30-35 mm Hg 6 Start D5W when BG approaches 250 mg/dL References: Krane EJ, Rockoff MA, Wallman JK, et al. Subclinical brain swelling in children during treatment of diabetic ketoacidosis. N Engl J Med 1985;312:1147-51 [Subclinical brain swelling may be a common occurrence during treatment of DKA in children.] Duck SC, Wyatt DT. Factors associated with brain herniation in the treatment of diabetic ketoacidosis. J Pediatr 1988;113:10-4 [Excessive secretion of vasopressin may exacerbate brain edema in children with DKA, and limitation of the rate of fluid administration may be prudent.] 2 Guideline for ED Management of DKA in Children Harris GD, Fiordalasi I, Harris WL, et al. Minimizing the risk of brain herniation during treatment of diabetic ketoacidemia: a retrospective and prospective study. J Pediatr 1990;117:22–31 [Failure of the serum sodium concentration to rise as glucose concentration declines is a marker for excessive administration of free water. An expanded repair period, with repair fluid containing an average of 125 mmol/L Na+ early in therapy, will usually protect against a downward trend in the concentration of sodium in serum and therefore against a rapid decline in effective serum osmolality. This regimen may be protective against neardeath episodes and brain herniation during treatment.] Rosenbloom AL. Intracerebral crises during treatment of diabetic ketoacidosis. Diabetes Care 1990;13:22-33 Bello FA, Sotos JF. Cerebral edema in diabetic ketoacidosis in children. Lancet 1990;336:64 Kaufman FR, Halvorson M. The treatment and prevention of diabetic ketoacidosis in children and adolescents with Type I Diabetes Mellitus. Pediatric Annals 1999; 28:576-82 [Review of DKA management.] Rosenbloom AL, Hanas R. Diabetic ketoacidosis (DKA): treatment guidelines. Clin Pediatr 1996;35:261–6 Mahoney CP, Vlcek BW, DelAguila M. Risk factors for developing brain herniation during diabetic ketoacidosis. Pediatr Neurol 1999;21:721-7 [In a retrospective review of children admitted in DKA, severity of acidosis and hypercapnea were the most reliable risk factors for brain herniation (none of the children who maintained a blood pH > 7.1 and a PCO2 > 20 mm Hg were affected). Rate of initial fluid administration in severe DKA was also a risk factor. Hydrating at a rate > 50 mL/kg during the first 4 hours was associated with an increased risk of brain herniation.] Muir A. Cerebral edema in diabetic ketoacidosis: a look beyond rehydration. J Clin Endocrinol Metab 2000;85:509-13 [The case is argued for a multifactorial cause of cerebral edema in DKA which cannot be reliably prevented by cautious rehydration protocols. Healthcare providers can reduce M&M by vigilantly watching for and responding to sentinel often subtle neurological signs and symptoms of cerebral edema, which precede often by hours the dramatic collapse typically described in these patients. Subclinical pathology causing increased ICP likely precedes initiation of therapy in almost all cases of DKA. Pathogenic mechanism for cerebral edema is unknown, but potential pathogenic contributors are hypoxia, vascular occlusion, cytotoxic effects of neuroexcitatory amino acids, and insulin-induced alterations of metabolism. Whatever the mechanism is, it must account for: occurrence with rare exceptions after onset of therapy; preponderance of children being affected; profound neurological dysfunction in the presence of minimal or no 3 Guideline for ED Management of DKA in Children radiologically visible cerebral edema; and initial pathological changes in the basal regions of the brain. The earliest clinical benefits of mannitol in the treatment of cerebral edema are not induced by a shift of fluid to the extravascular space, but rather a reduction of blood viscosity, thereby improving intracerebral blood flow.] Muir A. Do doctors cause or prevent cerebral edema in children with diabetic ketoacidosis? Pediatr Diabetes 2000;1:209-16 Felner EI, White PC. Improving management of diabetic ketoacidosis in children. Pediatrics 2001; 108:735-40 [A revised DKA protocol is offered which necessitates less fluid delivery and fewer calculations and is associated with more rapid correction of acidosis.] Dunger DB, Edge JA. Predicting cerebral edema during diabetic ketoacidosis. N Engl J Med 2001;344:302-3 Edge J, Hawkins MM, Winter DL, et al. The risk and outcome of cerebral edema developing during diabetic ketoacidosis. Arch Dis Child 2001;85:16–22 [A total of 34 cases of cerebral edema and 2940 episodes of DKA were identified, yielding a calculated risk of developing cerebral edema of 6.8/1000 episodes of DKA. This was higher in new (11.9 per 1000 episodes) as opposed to established (3.8 per 1000) diabetes. There was no sex or age difference. Cerebral edema was associated with a significant mortality (24%) and morbidity (35% of survivors).] Glaser N, Barnett P, McCaslin I, et al. Risk factors for cerebral edema in children and adolescents with diabetic ketoacidosis. N Engl J Med 2001;344:264–9 [Risk factors for cerebral edema in children with DKA are low PCO2, high serum BUN, & treatment with bicarbonate.] Dunger DB, Edge JA. Predicting cerebral edema during diabetic ketoacidosis. N Engl J Med 2001;344:302-3 [Caution must be used in interpreting the data of Glaser et al. (N Engl J Med 2001;344: 264-9) for the following reasons: (1) corrections were made in blood pH values, pCO2, and bicarbonate concentrations to account for collection of both arterial & venous blood samples; (2) bicarbonate may be a confounder (possible association with exposure, i.e. acidosis, and with outcome, i.e. cerebral edema). Based on this study, it would be difficult to justify the prohibition of bicarbonate, irrespective of severity of DKA. The data of Glaser et al. cannot be interpreted as revealing anything more than an association between severity and duration of DKA and risk of cerebral edema. The increased risk in new-onset diabetics and the occurrence of cerebral edema before treatment support the importance of other antecedent factors, such as accumulation of idiogenic, osmotically active substances that regulate cell volume, and individual biologic responses.] Marcin JP, Glaser N, Barnett P, et al. Factors associated with adverse outcomes in children with diabetic ketoacidosis-related cerebral edema. J Pediatr 2002;141:793-7 [After adjusting for potential confounding variables and the degree of neurologic 4 Guideline for ED Management of DKA in Children compromise at the initiation of therapy, intubation with hyperventilation is associated with adverse outcomes of DKA-related cerebral edema. Greater neurologic depression at the time of diagnosis of cerebral edema and a higher initial serum urea nitrogen concentration are also associated with poor outcome.] Chiasson JL, Aris-Jilwan N, Bélanger R, et al. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ 2003;168:859–66 [Diabetic ketoacidosis and the hyperglycemic hyperosmolar state are the most serious complications of diabetic decompensation and remain associated with excess mortality. Insulin deficiency is the main underlying abnormality. Associated with elevated levels of counterregulatory hormones, insulin deficiency can trigger hepatic glucose production and reduced glucose uptake, resulting in hyperglycemia, and can also stimulate lipolysis and ketogenesis, resulting in ketoacidosis. Both hyperglycemia and hyperketonemia will induce osmotic diuresis, which leads to dehydration. Clinical diagnosis is based on the finding of dehydration along with high capillary glucose levels with or without ketones in the urine or plasma. The diagnosis is confirmed by the blood pH, serum bicarbonate level and serum osmolality. Treatment consists of adequate correction of the dehydration, hyperglycemia, ketoacidosis and electrolyte deficits.] Carlotti APCP, Bohn D, Halperin ML. Importance of timing of risk factors for cerebral oedema during therapy for diabetic ketoacidosis. Arch Dis Child 2003;88: 170–3 [Cerebral edema is the most common cause of mortality and morbidity during the first day of conventional treatment for DKA in children. It is possible that therapy contributes to its development. Risk factors that predispose to cerebral edema should lead to an expansion of the intracellular and/or the extracellular fluid compartment(s) of the brain because water normally accounts for close to 80% of brain weight. With respect to the intracellular fluid compartment, the driving force to cause cell swelling is a gain of effective osmoles in brain cells and/or a significant decline in the effective osmolality of the extracellular fluid compartment. Factors leading to an expansion of the intracerebral extracellular fluid volume can be predicted from Starling forces acting at the blood-brain barrier. Some of these risk factors have an early impact, while others have their major effects later during therapy for diabetic ketoacidosis. Based on a theoretical analysis, suggestions to modify current therapy for DKA in children are provided.] Lawrence S, Pacaud D, Dean H, et al. Pediatric diabetic ketoacidosis. CMAJ 2003; 169:278-9 [The recommendation for fluid replacement in children with DKA is 5-10 mL/kg in the first hour, with higher rates used only in patients with significant hemodynamic compromise. Fluid replacement should be calculated over a 48-hour period. Use of bicarbonate is not routine for all pediatric patients with pH < 7.0. Intravenous bolus of insulin at the initiation of insulin therapy is not recommended.] Kamat P, Vats A, Gross M, et al. Use of hypertonic saline for the treatment of altered mental status associated with diabetic ketoacidosis. Pediatr Crit Care Med 2003;4:239-42 5 Guideline for ED Management of DKA in Children [HS 3% was used to control apparent intracranial hypertension with good clinical outcome and without apparent side effects in a small series of pediatric patients with DKA. Use of HS 3% has been shown to reduce raised ICP while augmenting intravascular volume and increasing MAP. The traditional therapy for cerebral edema in DKA has been the use of 20% mannitol. However, the brisk diuresis caused by this drug may lead to intravascular dehydration, hypokalemia, hypotension, prerenal azotemia, and even decreased cerebral blood flow. A small amount may also be converted to glycogen in the liver, thus further disturbing the glucose homeostasis.] White NH. Management of diabetic ketoacidosis. Rev Endocr Metab Disord 2003;4: 343-53 6 Guideline for ED Management of DKA in Children