Assignment-chap8
advertisement

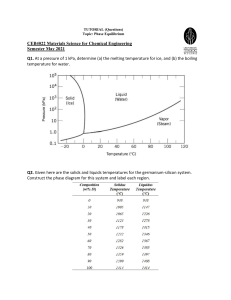
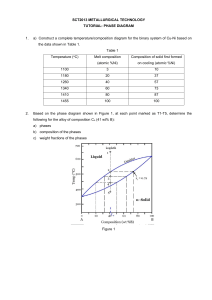
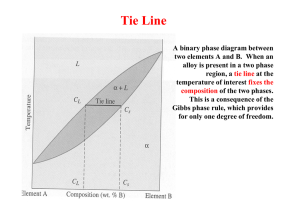
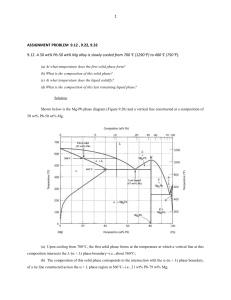
ASSIGNMENT FOR THE COURSE “ALLOYS AND PHASE DIAGRAMS” 8.8.3 In the binary system Ag-Cu (Fig. 8.19), what is the atomic concentration of Cu for the eutectic alloy? How does the liquid and the solid(s) composition vary with temperature during the solidification of an alloy with 80 at% Cu? ( at % ) 8.8.5 The maximum solubility of tin in copper in a bronze (Cu-Sn) is 15.8 wt% Sn at 586°C. What is the atomic fraction of the tin atoms at the solubility limit? 8.8.6 An alloy of the system Ag-Cu (Fig. 8.19) with 25 at% Cu is cooled down from the liquid state. What is the temperature at which the first solid phase is formed, and what is the type and the composition of this phase? At which temperature is this alloy completely solidified at equilibrium? What are the phases observed (and their compositions) at a temperature slightly lower than the complete solidification temperature? 8.8.7 With reference to the equilibrium diagram of the binary system Ni-Cu (Fig. 8.15), give: - the melting temperature (liquidus) of nickel. - the solubility of Cu in the solid phase of Ni at a temperature of 1250°C. Considering a Ni-Cu alloy with a concentration of Cu of 40 mol%, give: - the melting (liquidus) and solidification (solidus) temperatures - the phases present (with their composition) and their proportion at a temperature of 1300°C (Fig. 8.15) 8.8.9 Using the equilibrium phase diagram of SiO2-Al2O3 (Fig. 8.24), give the alloy with the lowest melting point. How many phased are present at equilibrium at the eutectic point? What is the weight concentration (wt%) of Si in this point? What is the solubility of Al2O3 in solid SiO2? Starting form the liquid state, describe the stages of formation of the alloy with 80 wt% Al2O3. (note the two axis wt% at the top and at% or mol% at the bottom of the figure)