Phase Diagrams Tutorial: Cu-Ni, Cu-Ag, Pb-Sn Alloys
advertisement
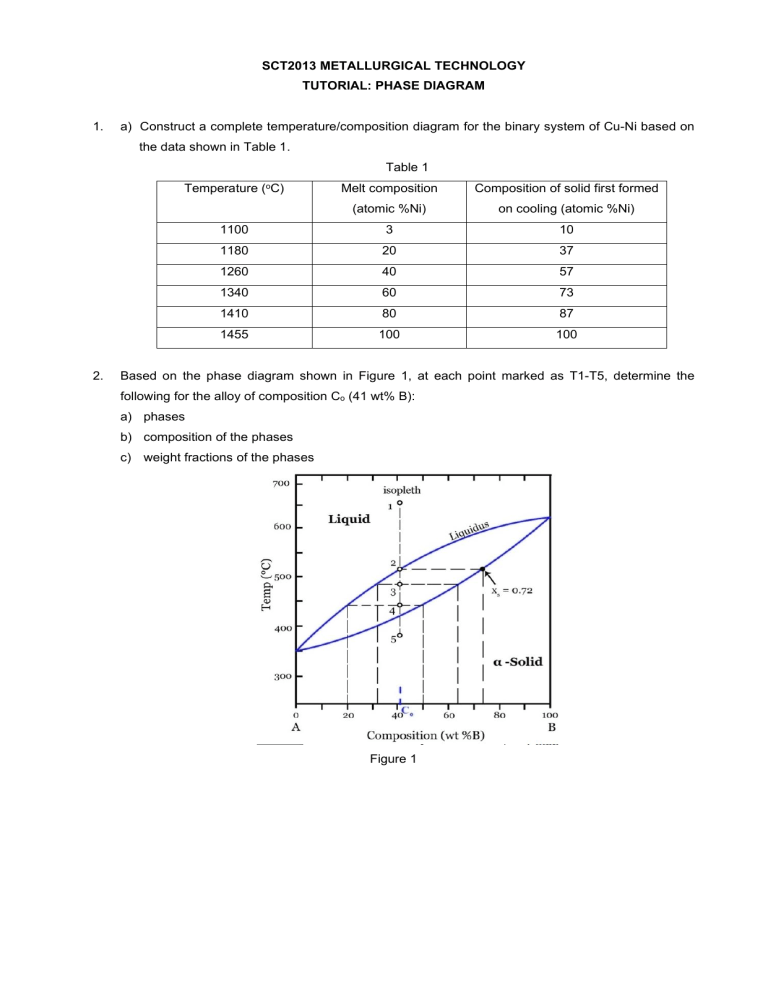
SCT2013 METALLURGICAL TECHNOLOGY TUTORIAL: PHASE DIAGRAM 1. a) Construct a complete temperature/composition diagram for the binary system of Cu-Ni based on the data shown in Table 1. Table 1 Temperature 2. (oC) Melt composition Composition of solid first formed (atomic %Ni) on cooling (atomic %Ni) 1100 3 10 1180 20 37 1260 40 57 1340 60 73 1410 80 87 1455 100 100 Based on the phase diagram shown in Figure 1, at each point marked as T1-T5, determine the following for the alloy of composition Co (41 wt% B): a) phases b) composition of the phases c) weight fractions of the phases Figure 1 3. Phase diagram of Cu-Ag is shown in Figure 2. Figure 2 a) A 90 wt% Ag alloy is heated to a temperature within the β + liquid phase region. If the composition of the liquid phase is 85 wt% Ag, determine: i. the temperature of the alloy ii. the composition of the β phase iii. the mass fractions of both phases b) For equilibrium solidification of a Cu-Ag alloy containing 40 wt% Ag, state the followings: i. the temperature at which solidification begins. ii. the temperature at which solidification is complete. 4. A 1.5 kg sample of 90 wt% Pb-10 wt% Sn alloy is heated to a 250 oC where at this temperature it is an entirely -phase solid solution. The alloy is to be melted to the extent that 50% of the specimen is liquid but the remainder being the phase. This may be accomplished either by heating the alloy or changing its composition while holding the temperature constant. The phase diagram of Pb-Sn system is shown in Figure 3. Figure 3 a) To what temperature must the specimen be heated? b) How much tin must be added to the 1.5 kg specimen at 250 oC to achieve this state?