TUTORIAL - UniMAP Portal
advertisement

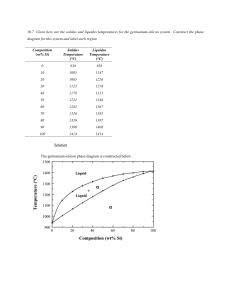
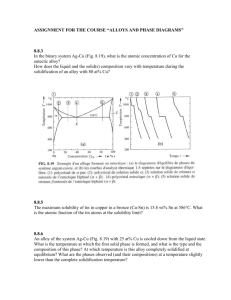
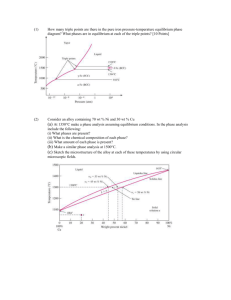
TUTORIAL 3 - Solution ENT 112 – Materials Engineering PHASE DIAGRAMS 1. Define (a) a phase in a material and (b) a phase diagram. Solution a) A phase in a material is a microscopic region that differs in structure and/or composition from another region. b) A phase diagram is a graphical representation of the phases present within a materials system for a range of temperatures, pressures and compositions. 2. Refer to the pressure-temperature equilibrium phase diagram for pure water below, calculate the degrees of freedom are there i) at the triple point? ii) along the freezing line? Solution i) ii) 3. At the triple point, there are zero degrees of freedom. Along the freezing line of pure water, there is one degree of freedom. In figure below, the pressure temperature phase diagram for H2O. Apply the Gibbs phase rule at point A, B, and C; that is, specify the number of degrees of freedom at each of the 1 points – that is, the number of externally controllable variables that need be specified to completely define the system Log. Pressure versus temperature phase diagram for H2O Solution Gibbs phase rule in general form is, P + F = C + N, for this system, the number of components, C, is 1, whereas N, the number of non-compositional variables, is 2--viz. temperature and pressure. Thus, the phase rule now becomes P + F = 1 + 2 = 3 or F = 3 – P where P is the number of phases present at equilibrium. At point A, only a single (liquid) phase is present (i.e., P = 1), or F = 3 – P = 3 – 1 = 2 which means that both temperature and pressure are necessary to define the system. At point B which is on the phase boundary between liquid and vapor phases, two phases are in equilibrium (P = 2); hence F = 3 – P = 3 – 2 = 1 or that we need to specify the value of either temperature or pressure, which determines the value of the other (pressure or temperature). At point C, three phases are present—viz. ice I, vapor, and liquid—and the number of degrees of freedom is zero since F = 3 – P = 3 – 3 = 0. Thus, point C is an invariant point (in this case a triple point), and we have no choice in the selection of externally controllable variables in order to define the system. 4. What is a binary isomorphous alloy system? Give an example & sketch its phase diagram Solution 2 The binary isomorphous alloy system is a two-component system in which the two elements are completely soluble in each other in the liquid and solid states and form a single type of crystal structure for all compositions. Example - The Cu-Ni system 5. Describe how the liquidus and solidus of a binary isomorphous phase diagram can be determined experimentally. Solution The liquidus and solidus of a binary isomorphous phase diagrams can be determined experimentally by measuring cooling rate for several specific alloy compositions and plotting the corresponding liquid-solid curves. The phase diagram can then be constructed by plotting the liquidus and solidus temperatures versus composition of the alloys. 6. Explain how a cored structure in 70% Cu-30% Ni alloy is (i) produced and (ii) eliminated by heat treatment? Solution: i) A cored structure is produced in a 70% Cu-30% Ni alloy when the alloy is cooled rapidly; without sufficient time for complete solid-state diffusion, concentration gradients remain in the alloy structure. 3 ii) 7. The cored structure can be eliminated in ingots and castings by heat treating at elevated temperatures. This homogenization process accelerates the required solidstate diffusion and thus produces a homogeneous structure in the alloy. Consider an alloy containing 70 wt % Ni and 30 wt % Cu in Figure below a) At 1350ºC make a phase analysis assuming equilibrium conditions. In the phase analysis include the following: (i) What phases are present? (ii) What is the chemical composition of each phase? (iii) What amount of each phase is present? b) Make a similar phase analysis at 1500ºC. c) Sketch the microstructure of the alloy at each of these temperatures by using circular microscopic fields. Solution: (a) i) ii) iii) The phases present are the liquid and solid (L + α). The chemical composition of liquid is wl = 62 wt % Ni while that of the solid is ws = 74 wt % Ni. The weight percent of solid and liquid are: (b) At 1500ºC, the alloy is 100% liquid. 4 (c) The microstructure of the alloy at these temperatures would look similar to the following sketches. 5