Tie Lines & Lever Rule: Phase Diagrams Explained
advertisement

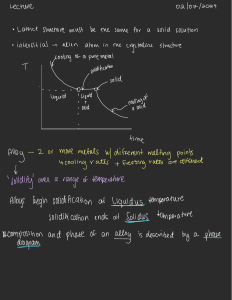
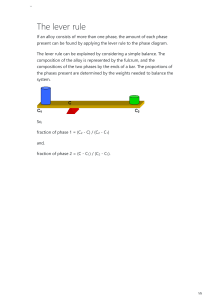
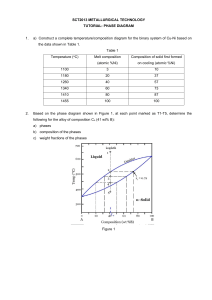
Tie Line A binary phase diagram between two elements A and B. When an alloy is present in a two phase region, a tie line at the temperature of interest fixes the composition of the two phases. This is a consequence of the Gibbs phase rule, which provides for only one degree of freedom. Lever Rule The Lever Rule is used to calculate the weight % of the phase in any two-phase region of the Phase diagram (and only the two phase region!) In general: • Phase percent = opposite arm of lever x 100 total length of the tie line For example, weight fraction, %X s = wo − wl x 100 ws − wl (of solid phase) ws − wo weight fraction, %X l = x 100 ws − wl (of liquid phase) Lever Rule When a material solidifies it does not have a constant concentration throughout the material but there will be concentration gradients, which will significantly alter the properties of the material. This is an important concept. In the example of Cu and Ni, the concentration of Ni that freezes at 1270 oC is 50 wt%, at 1250 oC is 45 wt% and 1200 oC is 40 wt%. Lever Rule Calculate the amount of α phase and L phase present in a Cu 40% Ni alloy at 1250 C In general: • Percent α phase = (% Ni in alloy) – (% Ni in L) % Ni in L - % Ni in α weight fraction, %X s = (of solid α phase) 40 − 32 x 100 = 62 % 45 − 32 weight fraction, % L = 38 % (of liquid phase) x 100 Solidification of a Solid-Solution Alloy The change in structure and composition of a Cu40% Ni alloy during equilibrium solidification showing that the liquid contains 40% Ni and the first solid contains Cu-52% Ni. At 1250 C, solidification has advanced and the phase diagram tells us that the liquid contains 32% Ni and the solid contains 45% Ni, which continues until just below the solidus, all of the solid contains 40% Ni, which is achieved through diffusion. Nonequilibrium Solidification and Segregation When cooling is too fast for atoms to diffuse and produce equilibrium conditions, nonequilibrium concentrations are produced. The first solid formed contains 52% Ni and the last solid only 25% Ni with the last liquid containing only 17% Ni. The average composition of Ni is 40% but it is not uniform.