Thermal equilibrium diagrams
advertisement
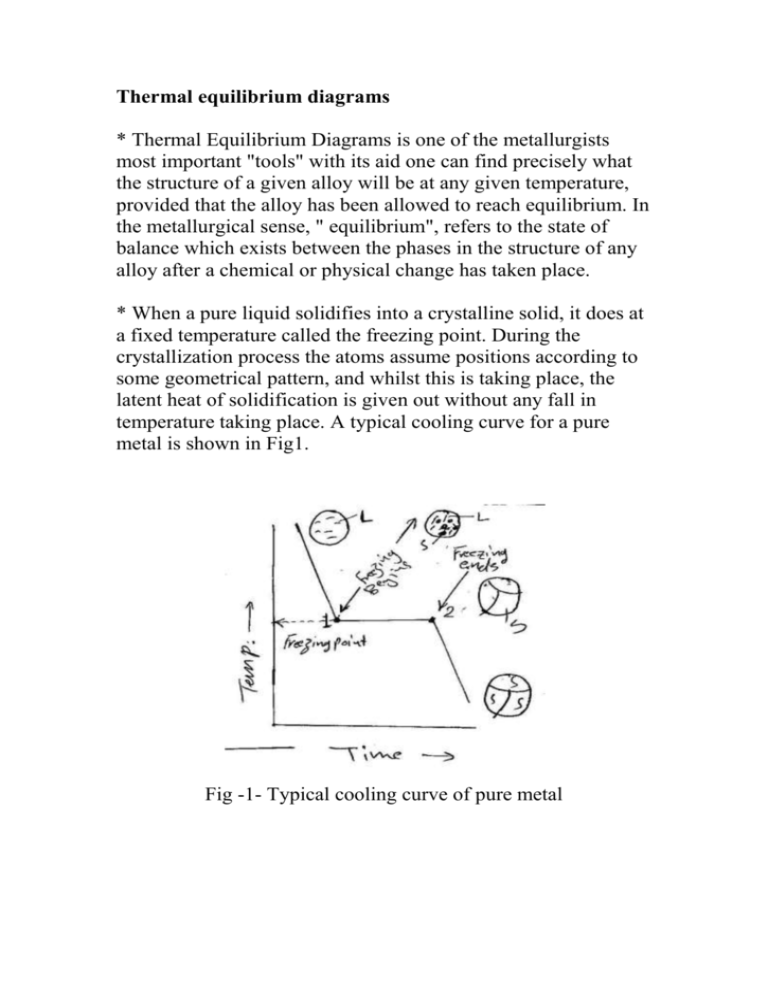
Thermal equilibrium diagrams * Thermal Equilibrium Diagrams is one of the metallurgists most important "tools" with its aid one can find precisely what the structure of a given alloy will be at any given temperature, provided that the alloy has been allowed to reach equilibrium. In the metallurgical sense, " equilibrium", refers to the state of balance which exists between the phases in the structure of any alloy after a chemical or physical change has taken place. * When a pure liquid solidifies into a crystalline solid, it does at a fixed temperature called the freezing point. During the crystallization process the atoms assume positions according to some geometrical pattern, and whilst this is taking place, the latent heat of solidification is given out without any fall in temperature taking place. A typical cooling curve for a pure metal is shown in Fig1. Fig -1- Typical cooling curve of pure metal Type -1- Two metals completely soluble in the liquid and solid states. * When an alloy solidify, it does over a range of temperature which will depend upon the composition of the alloy. Consider, for example, a number of alloy of different composition, containing the two metals A and B which form a series of solid solutions (see Fig.2) Fig -2- Thermal equilibrium of solid solution type using cooling curve For successive compositions containing different amounts of the metal A, freezing commences at a1, a2, a3, etc….. and ends at b1, b2, b3 , etc. Thus if we join all points a1, a2, a3, etc., we shall obtain a line called the liquids line, indicating the temperature at which any given alloy in the series will commence to solidify. Similarly, if we join the points b1, b2, b3 , etc., we have a line called solidus line, showing the temp, at which any alloy compositions represent completely homogeneous liquids. Solidus can be defined as the line below which all represented alloy compositions of A and B are completely solid. For temperature and compositions corresponding to the coordinates of points between the two lines both liquid solution and solid solution can exist together in equilibrium. Such a system occurs when two metals are soluble in each other in all proportions in both the liquid and solid states, such as (Cu+Ni) or (Au+Ag). There areas of diagrams are called "phase fields" and on crossing any sloping boundary line from one field to the next, the number of phases will always change by one. * At point (Q) Fig -3- in two-phase field both liquid and solid can exist together, if temperature horizontal is drawn through Q, the composition of the liquid solution is given by Y and the composition X. Q it self represents the overall composition of the mixture. Composition of solid ( ) = 20 % B + 80 % A Composition of liquid (L) = 80 % B + 20 % A * The relative amount of both liquid and solid at Q are given by the relative lengths Qx , Qy Amount of liquid Amount of solid 30 10% 50% 60 30 ( ) 100% 50% 60 Example Find the chemical composition at point (Y) in Fig -4- and the relative amount of both liquid and solid states. Solution At point (y) both ( L ) are exist - Composition of solid ( ) = 10 % B + 90 % A - Composition of liquid (L) = 26 % B + 74 % A - Amount of liquid = 10 100% 62.5% 16 - Amount of solid ( ) 6 100% 37.5% 16