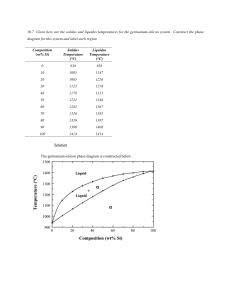
TUTORIAL (Questions) Topic: Phase Equilibrium CEB4022 Materials Science for Chemical Engineering Semester May 2021 Q1. At a pressure of 1 kPa, determine (a) the melting temperature for ice, and (b) the boiling temperature for water. Q2. Given here are the solids and liquids temperatures for the germanium-silicon system. Construct the phase diagram for this system and label each region. Q3. A copper–nickel alloy of composition 75 wt% Ni–25 wt% Cu is slowly heated from a temperature of 1300ᴼC (1573). (a) At what temperature does the first liquid phase form? (b) What is the composition of this liquid phase? (c) At what temperature does complete melting of the alloy occur? (d) What is the composition of the last solid remaining prior to complete melting? Q4. Derive Equations given below, which may be used to convert mass fraction to volume fraction. Q5. It is desirable to produce a copper-nickel alloy that has a minimum non cold-worked tensile strength of 350 MPa and a ductility of at least 48%EL. Is such an alloy possible? If so, what must be its composition? If this is not possible, then explain why.