Nitration of Methyl Benzoate
advertisement

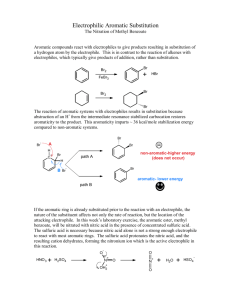
Heather Sopher Chem 36 Section 1 Experiment #2, Synthesis #1: Nitration of Methyl Benzoate February 20, 2007 Introduction: Electrophilic aromatic substitutions are reactions in which a hydrogen atom on an aromatic ring is replaced by an electrophile. Nitration reactions are very useful because the nitro group can be reduced to an amino group, which is a common benzene substituent. Methyl 3-nitrobenzoate will be synthesized by nitrating methyl benzoate. The starting material and product of the synthesis reaction will be analyzed by infrared spectrometry and melting point determination. Procedure: Concentrated sulfuric acid (0.5 mL) and concentrated nitric acid (0.5 mL) were added to a reaction tube and placed in an ice bath. Concentrated sulfuric acid (15 drops) was added to methyl benzoate (0.5 g) in a vial which was then packed in ice. While stirring, the cold H2SO4/HNO3 mixture was added drop-wise. After the acid mixture was added, the reaction mixture was removed from the ice to warm to room temperature, with stirring. It was then transferred by Pasteur pipet into a vial 1/3 full of ice and stirred for five minutes. A white solid formed, and was vacuum filtered with a Hirsch funnel. The crude product was recrystallized by adding a 50:50 mixture of water and ethanol drop by drop while heating the product. Slow cooling produced large white crystals. The mass, melting point and IR spectrum were obtained. Data, Analysis, and Calculations: Addition of cold H2SO4/HNO3 mixture to methyl benzoate (with 15 drops H2SO4) resulted in an oily and slightly cloudy solution. Transfer of the reaction mixture to vial filled with ice and stirring resulted in a cloudy solution and formation of white solid. Crude solid collected by vacuum filtration: 0.29 g of white crystals collected Melting point: 64 – 66 °C Percent yield: 44% Recrystallized solid collected by vacuum filtration: 0.27 g of large, white crystals collected Melting point: 66 – 68 °C Percent recovery: 93% IR spectrum of final product, identifying peaks (cm-1): 1376 1460 1739 Percent yield calculation: 0.5 g Me benzoate x 1 mol 136.15 g = 0.00367 mol methyl benzoate 0.00367 mol Me benzoate x 1 mol methyl-3-nitrobenzoate x 181.1476 g 1 mol Me benzoate 1 mol = 0.66 g methyl-3-nitrobenzoate 0.29 g x 100 = 44% 0.66 g Results and Discussion: Electrophilic aromatic substitutions involved the replacement of a proton on an aromatic ring with an electrophile that becomes a substituent. The electrophile, NO2+, is created in the following way: O O O N OH HO SO 3 H N O+ H O O H N+ O The solvent sulfuric acid protonates the methyl benzoate, creating the resonance stabilized arenium ion intermediate. The electron-deficient nitronium ion reacts with the protonated intermediate at the meta position. The ester group is a meta-deactivator, and the reaction takes place at the meta position because the ortho and para positions are destabilized by adjacent positive charges on the resonance structure. A proton from the ion is then transferred to the bisulfate ion to produce methyl-3-nitrobenzoate. The mechanism is as follows: CH3 O O HO C+ O CH3 + OH C O CH3 + HSO 4 + H2 SO4 HO C+ O CH3 HO O CH3 HO O CH3 + HO O CH3 + + HO C+ O CH3 HO Slow O 2N + C+ HO C+ HO O CH3 + O2 N H C+ O CH3 + + O2N H O CH3 O2 N H The reaction for the nitration of methyl benzoate is as follows: CH3 O O CH 3 O O HNO3 H 2SO 4 NO2 The final product of the reaction, methyl-3-nitrobenzoate, was obtained as large, white crystals in a 44% yield. The actual yield may have been higher but all of the white solid to be vacuum filtrated the first time could not be removed from the vial. Analysis by infrared spectroscopy shows the successful place of a nitro group on the aromatic ring. Peaks at 1376 cm-1 and 1460 cm-1 indicate that a nitro group is present. The peak at 1739 cm-1 indicates that the ester at the 1 carbon is still intact on the aromatic ring. Melting point determination of the final product was 66 – 68 °C. The literature melting point of methyl-3-nitrobenzoate is 78 °C. Conclusion: The goal of the lab was achieved, as methyl-3-nitrobenzoate was successfully synthesized by means of nitrating methyl benzoate. Analysis by infrared spectroscopy and melting point determination provided reinforcing data that the correct product was synthesized. References: ChemFinder.Com CambridgeSoft Corporation. <http://chemfinder.cambridgesoft.com/>. “Infrared Spectroscopy.” Penn State Erie, The Behrend College. CHEM 38. November 10, 2006. Mohrig, Jerry R., Christina Noring Hammond, and Paul F. Schatz. Techniques in Organic Chemistry. 2nd ed. New York, NY: W.H. Freeman and Company, 2006. Smith, Janice Gorzynski. Organic Chemistry. New York, NY: McGraw-Hill Companies, Inc., 2006. Williamson, K.L. Macroscale and Microscale Organic Experiments. 2nd ed. Boston, MA: Houghton Mifflin, 1994.