exp16nitration
advertisement

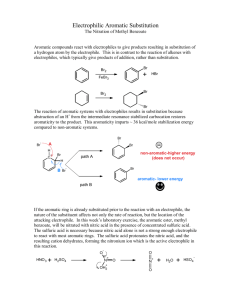
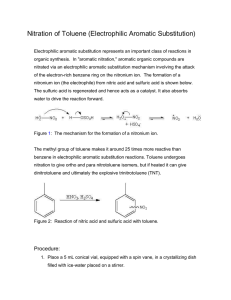
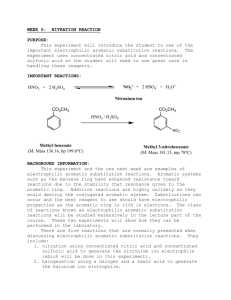
EXPERIMENT 16 NITRATION OF METHYL BENZOATE Aromatic compounds undergo numerous electrophilic substitution reactions. Nitration is an excellent example of aromatic electrophilic substitution. It is also a very common tool for introducing a nitrogen atom into an aromatic compound for synthetic purposes. Electrophilic aromatic substitution involves (1) creating an electrophile, (2) attack by the electrophile on the aromatic nucleus, and (3) elimination of H+ to return to an aromatic system. In the case of nitration, the electrophile which attacks the aromatic nucleus is NO2+, the nitronium ion. It is generated by the reaction between concentrated sulfuric and concentrated nitric acids (Eq.1). (Eq.1) 2H2 SO4 + HNO3 ----> H3O+ + NO2+ +2HSO4 – This electrophile may also be generated by the auto ionization of concentrated nitric acid (Eq.2). A much lower concentration of NO2+ is obtained in this case. (Eq.2) 2HNO3 ------> HO2+ + NO3- + H2O Which method is used to generate the electrophile depends upon the reactivity of the aromatic substrate toward electrophilic substituents. The reactivity the reactivity of an aromatic compound (Ar-G) depends on the nature of the substituent group, G. Electron-releasing substituents increase reactivity while electron-withdrawing substitution decrease reactivity. The SLOW STEP in electrophilic aromatic substitution is the formation of a resonance stabilized cation. Electron-release makes this cation more stable and easier to form. Electron withdrawal has the opposite effect. Strong, moderate, and weak activators direct substitution to the ortho and para positions. Deactivators direct substitution to the meta position. Halogens deactivate slightly, but direct substitution to the ortho and para positions. Deactivators direct substitution to the meta positions. These directing properties of substituent groups are also related to the stability of the cation produced. The following general list of substituent groups and their effect on both reactivity and orientation of reaction is useful: Strong Moderate Week Activators (o,p directors) -NH2 -NHR, -NR2, OH(amines and phenols) -NHCOR, -OR, -OCO-R (amides, ethers, esters) -R, -Ar (alkyl, phenyl) WEAK DEACTIVATORS; o/p DIRECTORS -Br, -Cl, -I (halides) Deactivators (meta directors -NR3+, -NO2, -CN (ammonium, nitor, nitriles) -CO-H, -CO-R, -COOH, -COOR, (aldehydes, ketones, acids, esters) In this experiment, the compound methyl benzoate is to be nitrated. Methyl benzoate is an ester. -COOCH3 is attached to the benzene ring resulting in STRONG DEACTIVATION. Because the ester group is a strong deactivator, Nitro group is introduced to META POSITION of methyl benzoate to produce 3nitromethylbenzoate, as shown by (Eq.3) and (Eq. 4) O O OCH 2 OCH 3 ( Eq.3) + + NO2+ H NO 2 O O OCH 2 (Eq. 4) + OCH 3 H NO 2 + H+ NO 2 PROCEDURE 1. To a clean and dry test tube, add about 0.500 g of methyl benzoate (it is a liquid). Then add 15 drops of concentrated sulfuric acid. Mix the two liquid well (There should be only one layer after mixing). Then put the test tube in a beaker containing ice to cool down for about 2 minutes. 2. In a separate clean and try test tube, add 15 drops of nitric acid and 15 drops of concentrated sulfuric acid, mix well. 3. Use a dropper, add the mixture of nitric acid and sulfuric acid into the first test tube (containing methyl benzoate and sulfuric acid), keep the reaction mixture in ice. Mix the reaction mixture with stirring rod for two minutes. The color of reaction mixture should be light yellow and it is a homogenous liquid mixture. 4. Take the reaction test tube out off the ice, warm to room temperature. Keep at room temperature for 5-10 minutes. 5. Pour the reaction mixture into 5-10 gram of ice in a small beaker, stir. The white solid product should be formed. 6. Suction filtering the solid product. Wash it with water. 7. Pressing dry the solid product and weigh it. EXPERIMENT 16: Report and Worksheet NITRATION OF METHYLK BENZOATE Student Name: ___________________ Day: _____________ DATA mass of methyl benzoate used ______ grams______moles mass of m-nitromethylbenzoate obtained ______grams melting range observed ______ Ref. 78oC THEORETICAL YIELD 3-NITROMETHYLBENZOATE _______grams (show calulations) PERCENT YIELD: __________________ QUESTIONS 1. Why does methyl benzoate dissolve in concentrated sulfuric acid? 2. What is the EQUATION for the reaction between concentrated nitric and sulfuric acids? 3. What is the DOT STRUCTURE for NO2+? 4. What is the MECHANISM for the formation of m-nitromethylbenzoate? 5. Why does reaction occur at the META position only? 6. What is the PURITY of the m-nitromethylbenzoate recovered based on its melting range?