Nitration of Aromatics: EAS & Methyl Benzoate
advertisement

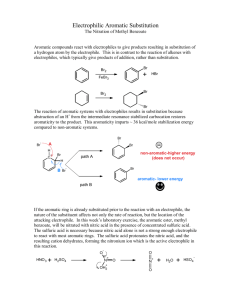
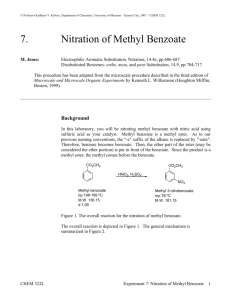
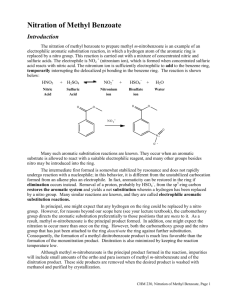
11/30/2012 Nitration of Aromatics CH3 CH3 O2 N NO2 dynamite (Acme) toluene NO2 tri-nitrotoluene (TNT) ? Nitration of Methyl Benzoate A Study of Electrophilic Aromatic Substitution 1 11/30/2012 Nitration of Methyl Benzoate CO2CH3 CO2CH3 HNO3, H2SO4 NO2 Nitration can introduce a nitro group into three different positions on the aromatic ring. Mechanism of EAS 1) Formation of Reactive Electrophile • Differs for each substituent being added • Electrophile for nitration is +NO2 2) Reaction of Electrophile with Aromatic Ring 3) Deprotonation to Regenerate Aromatic Ring Steps 2) and 3) are essentially identical for all EAS reactions. 2 11/30/2012 Steps in EAS H H E+ E Deprotonation by a base at the tetrahedral The reaction of the reactive electrophile carbon containing the electrophile results with the aromatic ring gives a high energy in reformation of a stabilized aromatic ring carbocation intermediate. This carbocation with a new substituent (E) present. is stabilized by resonance, but the aromatic stabilization has been lost. Steps in EAS H H E+ E E Y H + H-Y Y H E Product resulting from simple addition of E+ and Y - does not occur because the addition product is not aromatic. 3 11/30/2012 Nitration via NO2+ The reactive electrophile in nitration is NO2+ OH O N O H OSO3H OH2 O O N N O O + H2O In the first step, nitric acid acts as a base and is protonated by the stronger sulfuric acid. Then loss of water gives the nitronium ion, NO2+ Nitration of Benzene first step is the reaction of the by The carbocation is then deprotonated electrophilic nitronium with the water, the strongest baseion present in the nucleophilic pi electrons in the aromatic sulfuric acid/nitric acid mixture. ring to form a high-energy carbocation. 4 11/30/2012 EAS with Substituted Benzenes Substituents can cause a compound to be either more or less reactive than benzene Substituents affect the orientation of the reaction – the site of attack is affected EAS with Substituted Benzenes 5 11/30/2012 Positional Selectivity Y Y E+ Y Y E E ortho-product meta-product E p ara-product Three positional isomers are possible, but they are never formed in equal amounts The properties of the substituent Y controls the selectivity. Positional Selectivity All electron-donating (activating) groups give predominantly the ortho- and paraisomers as products (see red subst. above) 6 11/30/2012 Positional Selectivity All strongly electron-withdrawing (deactivating) groups give predominantly the meta- isomer as product (see blue subst. above) Positional Selectivity The weakly deactivating halogens give predominantly the ortho- and para- isomers as product (see green subst. above) 7 11/30/2012 Activating ortho/para directors The electron donation of activating groups is greatest for the ortho- and para- intermediates (and transition states). Deactivating meta directors The electron withdrawal of deactivating groups is greatest for the ortho- and paraintermediates (and transition states), thus making the meta pathway lowest energy. 8 11/30/2012 Halogens The inductive effect of the electronegative halogens leads to reduced reactivity, but the lone pairs on the halogen still make the ortho and para pathways faster than the meta pathway. Methyl Benzoate O OCH3 C O OCH3 C Is the –CO2Me group an activating or deactivating group? Which product(s) do we expect to predominate? 9 11/30/2012 Procedure suggestions Sulfuric acid will be the solvent for the methyl benzoate. Cool it in the ice bath before adding the methyl benzoate. The methyl benzoate is in dropper bottles. Weigh out about 3.4 g and add to the cooled sulfuric acid. Do NOT add the nitrating acid mixture all at once! Add drop wise over about a three minute period. Procedure suggestions (Cont.) You need to have the ice melted before you filter to isolate your crystalline product. Develop your TLC plate to near the top (less than 10 mm) of the plate; you need to distinguish between the isomeric products. “Mother liquor” is the solvent (filtrate) that passed through the filter in isolating the recrystallized product – should contain more impurities and minor products. 10 11/30/2012 Safety Be extremely careful with the strong acids used in this experiment. They will rapidly burn skin! Be careful with the autodispensers in the hood. Do not touch the tips with hands or arms. Be careful not to dispense too rapidly. Wipe up all spills outside the dispensing tray. Use safety showers immediately for any spill of acid on clothing. Two students of same gender should bring fire blanket to shower to act as modesty curtain; other students should leave lab. Safety Avoid contact with the organic products in this reaction. They are strong irritants. Clamp your recrystallization flask in the ice bath or it will overturn. Clamp your filter flask! Read the safety box in the lab manual at the end of the experimental section for this experiment again before starting this experiment. 11 11/30/2012 Clean-up Procedures Wipe all spills of acids with a wet paper towel and then with bicarbonate solution. Wipe your bench area with a moist paper towel before you leave the lab. There are TWO waste bottles – one for organic waste and one for aqueous waste (don’t mix!). TLC plates and filter paper also go into waste containers. Univ. of Maryland Organic II Lab Explosion – Sept. 26, 2011 Waste Container hood after explosion and fire when 2 students poured nitrating acid into organic waste container!!! • ALWAYS pour waste into the appropriate container. • You will not have waste nitrating acid in today’s lab because you will be given EXACTLY the correct amount from an autodispenser! 12