Experiment E. Electrophilic Aromatic Substitution
advertisement

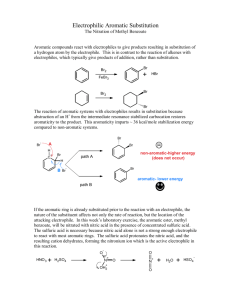
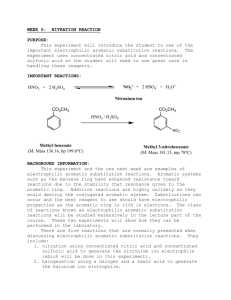
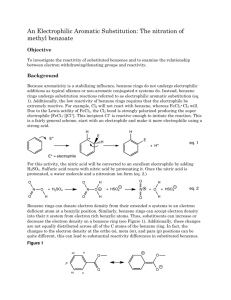
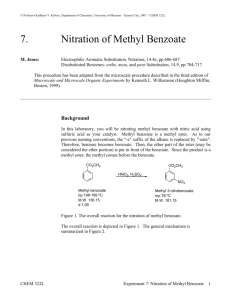
Organic Chemistry I Lab CHEM 2211L School of Science & Technology Georgia Gwinnett College Experiment E. Electrophilic Aromatic Substitution ______________________________________________________ 1. EXPERIMENTAL OBJECTIVES: a. Conduct an electrophilic aromatic substitution reaction on microscale level. b. Isolate the product. c. Characterize and analyze the product. d. Present, analyze, and discuss results in a written lab report. 2. SPECIAL INSTRUCTIONS: a. Before you will be allowed to begin the experiment, you must have completed lab report sections up to procedures as described in your Instructions for Students in Organic Chemistry (ISOC). All procedures, data, and observations must be recorded directly into the laboratory notebook as part of the report. . b. Chemicals and solvents: (1) methyl benzoate [ ] (2) concentrated sulfuric acid (3) concentrated nitric acid (4) methanol (5) hexane (6) ether c. Lab quiz at the beginning of the first lab session. You should have your notebook completed up to the “procedures” section before you come to the first lab session. d. There are a number of calculations for this lab. All calculations go into the lab notebook. Remember to label numbers with units and watch significant figures. 3. PROCEDURES: Introduction: In this experiment, we will apply the theory of electrophilic aromatic substitution that we learnt in class, by the preparation of methyl m-nitrobenzoate from the benzene derivative methyl benzoate via a nitration reaction. This is an important example of the type of reaction that comes under the umbrella of ‘electrophilic aromatic substitution (EAS) reactions’. We will purify the product, and then analyze its purity by TLC and melting point. Page 1 of 4 2/12/16 Organic Chemistry I Lab CHEM 2211L School of Science & Technology Georgia Gwinnett College The -electrons in the aromatic ring are nucleophilic in this reaction (as they are in all EAS reactions). An intermediate “arenium ion” that is no longer aromatic will be formed. Because aromatic rings are particularly stable, it takes a very strong electrophile to disrupt the aromaticity of the starting material. Often a Lewis acid (such as AlCl3 or FeBr3) or a Brønsted acid (such as H2SO4) is required to catalyze the reaction. The cation that we generate in this reaction is called the nitronium ion (NO2+) and it is sufficiently electrophilic so that no catalyst is needed. Reaction: Draw the structures of the reactant methyl benzoate, as the electrophile (the nitronium ion,), and the product in your lab book. Hypothesize as to what position(s) the nitrornium will add: ortho, para, meta. Draw out the mechanisms for how the nitronium ion is formed from sulfuric acid. Then draw out the mechanism for how the nitronium ion is added to the methyl benzoate. Include ALL electron-pushing arrows, all lone pair electrons, all formal charges, and ALL possible resonance structures. Procedure: Safety Precaution: Use care when handling concentrated sulfuric and nitric acids. Nitric acid reacts vigorously with many organic compounds. Additions of nitric acid to organic compounds or vice versa must be done under very carefully controlled conditions. Any acid spills are to be neutralized with sodium bicarbonate powder and cleaned up immediately. Place methyl benzoate (0.4 g) in a 10 mL round bottomed flask, along with a small magnetic flea stir bar. Cool the liquid in an ice bath using a 100 mL beaker on a magnetic stir plate to provide continuous stirring. Add 0.6 mL of concentrated sulfuric acid to the flask, using a glass Pasteur pipette. After allowing the mixture to cool for a minute, add 0.3 mL of concentrated nitric acid very slowly dropwise, with continuous stirring. This addition should take about 5 minutes. After the addition is complete, let the reaction stir in the ice bath for 15 minutes, and then allow it to warm to room temperature. Remove the stirrer bar from the solution at this point. Isolation: Pour the reaction mixture onto ~ 3.0 g of ice in a 50 mL beaker. Wash out the rest of your product from the reaction flask using ice-cold water. After stirring for approximately 5 minutes, vacuum filter the mixture. Wash the solid with 2 portions of ice-cold water, each time using enough water to cover the solid in the Page 2 of 4 2/12/16 Organic Chemistry I Lab CHEM 2211L School of Science & Technology Georgia Gwinnett College Buchner funnel. Leave the sample to dry on the vacuum until dry to the touch with a spatula. Obtain a weight of your crude product (with this you can calculate a crude % yield), and a melting point range. Save a small amount of your crude product (about ½ the size of a grain of rice) for TLC analysis (see later). Purification & Analysis: Recrystallize your crude product from a minimum amount of hot methanol (the solution can be heated on a hot plate), and isolate the product (which should be considerably more crystalline after purification) via vacuum filtration as before. Leave your pure product to dry for about 15 minutes (test dryness with a spatula), and then obtain the weight to use in your calculations of your final % yield. Measure the melting point range of your purified product. What should the melting point be, based on your UTORP? Run a TLC analysis of both your crude and recrystallized samples (on the same TLC plate), by dissolving a small amount of each sample in ether, Use an 80:20 hexane:ether solvent system. Mark the positions of any spots with a pencil. Place your dry, recrystallized product in the waste container as directed by instructor. Conclusion Questions: (1) Based on the mechanism for the formation of the NO2+ electrophile, which would you say is the stronger acid: nitric or sulfuric acid? Provide a source of information which backs up your answer and cite the source. (2) Provide a rationale as to why the nitronium added onto the methyl benzoate in the position that it did. Was your hypothesis supported by the data collected in lab? If not, then explain why the data did not support your hypothesis. (3) How many different, or non-equivalent hydrogen atoms are there in methyl benzoate? How many are in the final product? (4) Explain why your crude % yield differs from your final % yield and what factors could have affected this difference. (5) Put a table like the one shown below in your report, and use this to correctly identify the product you obtained. Show how the melting point of your product confirms that you did, indeed, produce the expected isomer. Page 3 of 4 2/12/16 Organic Chemistry I Lab CHEM 2211L isomer IUPAC name School of Science & Technology Georgia Gwinnett College Structure Literature Observed m.p. (C) m.p. (C) ortho meta para Reaction Product Page 4 of 4 2/12/16