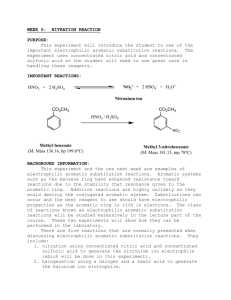
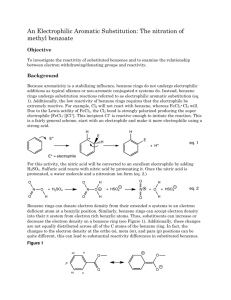
Nitration of Methyl Benzoate: Lab Experiment
advertisement
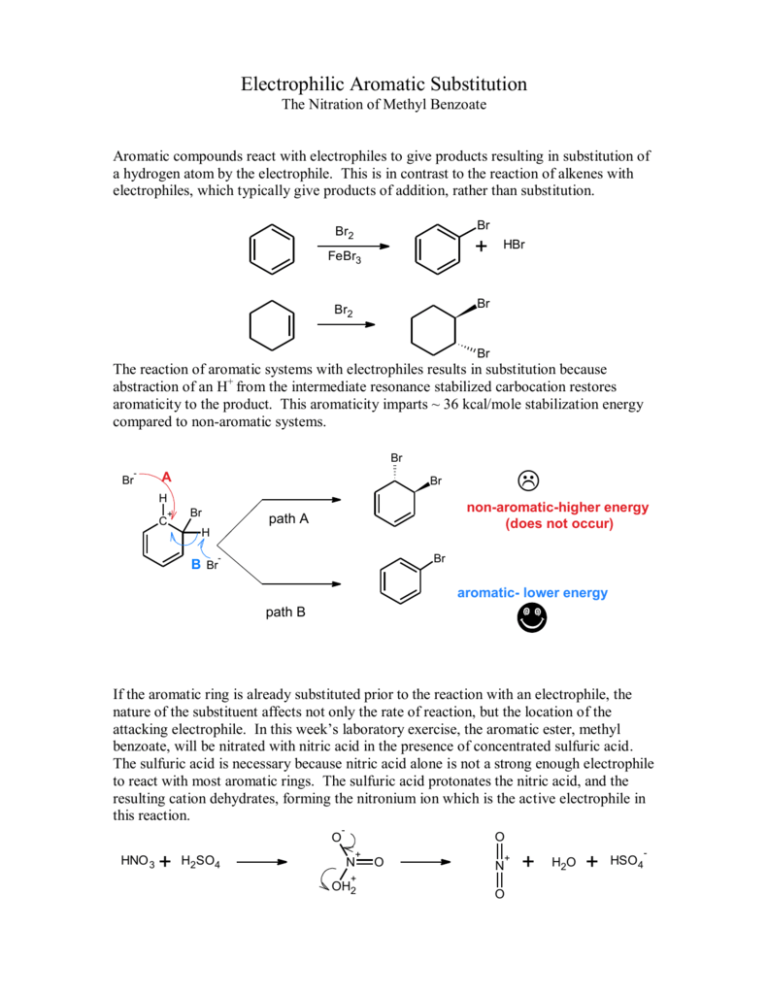
Electrophilic Aromatic Substitution The Nitration of Methyl Benzoate Aromatic compounds react with electrophiles to give products resulting in substitution of a hydrogen atom by the electrophile. This is in contrast to the reaction of alkenes with electrophiles, which typically give products of addition, rather than substitution. Br Br2 + FeBr3 HBr Br Br2 Br The reaction of aromatic systems with electrophiles results in substitution because abstraction of an H+ from the intermediate resonance stabilized carbocation restores aromaticity to the product. This aromaticity imparts ~ 36 kcal/mole stabilization energy compared to non-aromatic systems. Br - Br A H + C Br Br non-aromatic-higher energy (does not occur) path A H Br - B Br aromatic- lower energy path B If the aromatic ring is already substituted prior to the reaction with an electrophile, the nature of the substituent affects not only the rate of reaction, but the location of the attacking electrophile. In this week’s laboratory exercise, the aromatic ester, methyl benzoate, will be nitrated with nitric acid in the presence of concentrated sulfuric acid. The sulfuric acid is necessary because nitric acid alone is not a strong enough electrophile to react with most aromatic rings. The sulfuric acid protonates the nitric acid, and the resulting cation dehydrates, forming the nitronium ion which is the active electrophile in this reaction. O HNO 3 + H2SO4 - O + N O + N + OH2 O + H2O + HSO4 - The nitronium ion reacts with methyl benzoate to produce the nitrated methyl benzoate as shown below. Note that the position of the nitro group is unspecified in the drawing (the nitro is shown attached to the aromatic ring, but the location of the bond is not specified). O OCH 3 O NO 2 OCH 3 + NO 2 Based on the stability of the resulting intermediate, draw the expected product of the nitration of methyl benzoate as part of your pre-lab activity in the introduction to your lab report. Also include the physical and chemical properties of methyl benzoate, concentrated sulfuric acid, concentrated nitric acid and methanol. Methyl benzoate is found naturally and is known as niobe oil, cananga, or ‘harsh wintergreen’. It occurs naturally in various plants, including clove and black current. You may find the aroma familiar and pleasant, but the concentrated material can irritate the mucous membranes and skin. This experiment consists of four major parts: 1. The nitration of methyl benzoate 2. Isolation of crude methyl nitrobenzoate (you should provide the required number indicating the position of the nitro group) 3. Purification of the crude product 4. Characterization of the purified product. (mp, IR) Experimental Details Safety: 1. Concentrated nitric acid is a strong oxidizer as well as a strong acid. Therefore it is corrosive to skin, eyes, and mucous membranes. Contact with skin will result in the formation of a yellow or yellow-brown color on the affected part which will not be removed by washing. 2. Concentrated sulfuric acid is corrosive to skin, eyes, and mucous membranes 3. Methanol is toxic, both by ingestion and inhalation. Procedure: 1. Place 12mL concentrated sulfuric acid in a 125mL Erlenmeyer flask and cool the contents in an ice bath until the temperature is <5oC. 2. Add 6.1mL methyl benzoate (calculate the amount in grams) to the contents of the flask described in step 1. Cool the contents to 5oC in an ice bath. 3. Place 4.0mL of concentrated sulfuric acid in a 25mL Erlenmeyer flask and cool the contents in a beaker of ice water. Add 4.0 mL of concentrated nitric acid to the sulfuric acid and keep the mixture of acids in the ice bath. 4. Using a Pasteur pipette, add the mixed acid reagent dropwise to the contents of the 125mL Erlenmeyer flask. Swirl the contents of the flask often to ensure good mixing. Maintain the temperature during addition between 5o and 15oC. (The reaction is exothermic and must be controlled by cooling and slow addition rate) 5. After the addition of the nitric acid/sulfuric acid mixture is complete, remove the 125 mL Erlenmeyer flask from the ice bath and allow the reaction mixture to warm to room temperature. 6. After 15 minutes, pour the reaction mixture into a 250mL beaker containing ~50g ice chips. After the ice has melted, isolate the solids by suction filtration. DO NOT WASH THE PRODUCT UNTIL STEP 7 IS DONE. 7. Transfer the acidic filtrate into the ‘waste acid’ container in the hood. Then replace the filter on the filter flask and continue with step 8. 8. Wash the isolated solid with 20 mL cold water; the clear filtrate can be disposed in the sink with running water. 9. Wash the solids with 10mL portions of cold methanol. Dispose of the filtrate in the ‘waste organic’ container in the hood. DO NOT COMBINE WITH THE WASTE ACID. 10. Save a small portion of ‘crude’ product for a melting point determination. 11. Weigh the remainder of product and recrystallize from an equal weight of methanol. Warm the methanol / product mixture on a hot plate until solution is achieved, then remove from heat. Cool the solution in an ice bath. 12. Isolate the purified product by suction filtration. Air dry for ~10 minutes, then place the crude material in an oven at 60oC. Dispose of the filtrate in the ‘waste organic’ container. 13. Weigh the dried material and obtain a melting point and IR. 14. Calculate the per cent yield for the purified material. Questions to be addressed as part of your conclusion: What evidence do you have that the purification procedure was effective in increasing the purity of your product? Estimate the yield loss that occurred as a result of the purification steps. What caused this loss? Does the spectral data support the proposed structure of your product? Would you expect the nitration of phenol to give the same substitution pattern? Why or why not? Created 1/2/06 by J. Neilan Updated 3/11/07