THIOALCOHOLS AND DISULFIDES:
advertisement

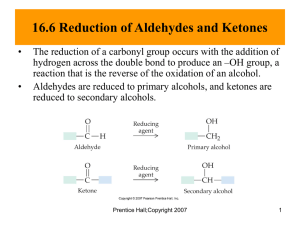
Organic chemistry and Biological chemistry for Health Sciences 59-191 Lecture 10 THIOALCOHOLS AND DISULFIDES: Derivatives of hydrogen sulfide, H-S-H are called thioalcohols or mecaptans (R-S-H). Sulfur counterpart of ethers is called thioethers (R-S-R/, replacement of oxygen atom by a sulfur atom). The –SH group is called the thiol group, the mercaptan group, or the sulfhydral group. Some very important properties of proteins depend on this group. Thioalcohols can be oxidized to disulfides, compounds whose molecules have two sulfur atoms joined by a covalent bond. R-S-H + H-S-R + [O] 2CH3SH + [O] R-S-S-R + H2O CH3 –S-S-CH3 + H2O Disulfides occur widely among proteins. It is very easily reduced to thiols. R-S-S-R + 2[H] CH3 –S-S-CH3 + 2[H] R-S-H + H-S-R 2CH3SH ALDEHYDES AND KETONES: All simple sugars are either polyhydroxy aldehyde or polyhydroxy ketones. Both aldeyde and ketone contain the carbonyl group (C=O). The carbon in C=O must bond to at least one H in an aldehyde. The other single bond must be to carbon or another H but not to O, N,or S. Thus all aldehyde have CH=O group, called the aldehyde group. Carbonyl carbon in ketone is joined to two other carbons. Only then carbonyl group is called a keto group. Structure of ketone is sometimes condensed to RCOR/. PHYSICAL PROPERTIES OF ALDEHYDES AND KETONES: Aldehyde and keto groups are unsaturated and planar. The carbonyl group makes aldeheyde and ketones polar compounds (Oxygen is more electronegative than carbon). Due to their polarity they have higher boiling point than alkanes with comparable formula mass. Oxygen of carbonyl group can accept hydrogen bonds which makes aldehydes and ketones relatively water soluble. NAMING OF ALDEHYDE: Select longest carbon chain with aldehyde group Name the parent by changing the –e ending of the corresponding alkane to –al Number the carbon chain to the give the carbon atom of the carbonyl group number 1. Assemble the rest of the name in the same way that is used to naming alcohols, except do not include “1” to specify the location of the aldehyde group. IUPAC RULES TO NAME KETONES: Naming ketones are identical to those for the aldehydes, except for two obvious changes. The name of the parent ketone must end in –one The keto group must be located by a number. In the numbering of chain, the location of the keto group takes precedence, not the location of the substituents. Quite often simple ketones are given common names that are made by naming the two alkyl group attached to the carbonyl group and then following these names by the word ketone. Oxidation of aldehydes and ketones: The aldehyde group is easily oxidized to carboxylic acid group by even mild reagents. So some very simple test-tube tests are available to check the presence of aldehyde group. TOLLENS’ TEST: One very mild oxidizing reagent, called Tollen’s reagent, consists of an alkaline solution of the silver ion in combination with two amonia molecules [Ag(NH3)]+, oxidizes the aldehyde group to a carboxyl group (rather to its anion form), and the silver ion is reduced to metallic silver. When this reaction occurs in a thoroughly clean, grease free test tube, the silver plates to the glass as a beautiful mirror. This test is called Tollen’s test. This test is used to check the presence of aldehyde group in an unknown compound. RCH=O (aq) + 2[Ag(NH3)2]+(aq) + 3OH-(aq) RCOO- (aq) + 2Ag (s) + 2H2O + 4NH3 (aq) Benedict’s test: Benedict’s reagent, another mild oxidizing agent, consists of a basic solution of the copper (II) ion and the citrate ion react similarly with aldehyde. The copper reagent is deep blue. When it reacts with aldehyde a brick red precipitate of Cu2Ois formed. RCH=O (aq) + 2Cu[citrate]2+ (aq) + 5OH- (aq) RCO2- (aq) + Cu2O (s) + 3H2O + 2[Citrate] Alpha hydroxy aldehyde, alpha keto aldehyde and alpha hydroxy ketone which occur in various carbohydrates, give positive benedict’s tests. So Benedict’s test has long been used to detect glucose in urine for medical diagnosis. REDUCTION OF ALDEHYDES AND KETONES: Aldehydes are reduced to primary alcohols and ketones to secondary alcohols by the direct addition of hydrogen or by hydride ion transfer. Under heat and pressure and in the presence of a finely divided metal catalyst, the carbonyl group of aldehyde and ketones add hydrogen according to the following equations In living cells H:- can be supplied by a donor that transfers it directly to the acceptor. One hydride acceptor is the carbonyl group. When an aldehyde or keto group accepts H :-, the following reaction occurs and the anion of an alcohol forms. We will represent a donor by the symbol Mtb:H where Mtb refers to a metabolite, a chemical intermediate in metabolism. The alcohol anion is a strong proton acceptor. So newly formed anion instantaneously takes a proton either from a water molecule or from some other proton donors in the surrounding buffer system. One of the many examples of hydride ion reduction in cells is one of the steps in the metabolism of glucose. Reaction of aldehyde and ketones with alcohols: Alcohols add to the carbonyl group of aldehydes and ketones to form hemiacetals and hemiketals respectively. Hemiacetals contain both an alcohol and ether functional group in the same carbon. The two groups are so close to each other that they modify each other’s properties. So we have neither an ordinary alcohol nor ordinary ether. Unlike either alcohol or ether, hemiacetals are not very stable. They readily break down to alcohols and aldehydes. The hemiacetal system is stable when it occurs among carbohydrates. F.example. Glucose molecules exist as a cyclic hemiacetals. Ketones react with alcohols, similarly to the way aldehydes do, to form hemiketal. Hemiketals are even less stable than hemiacetals. But hemiketal system does occur among carbohydrates.