16.7 Addition of Alcohols: Hemiacetals and Acetals
advertisement

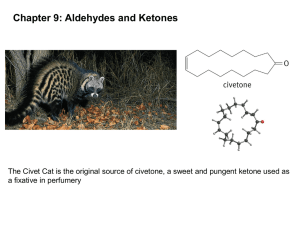
16.6 Reduction of Aldehydes and Ketones • • The reduction of a carbonyl group occurs with the addition of hydrogen across the double bond to produce an –OH group, a reaction that is the reverse of the oxidation of an alcohol. Aldehydes are reduced to primary alcohols, and ketones are reduced to secondary alcohols. Prentice Hall;Copyright 2007 1 16.7 Addition of Alcohols: Hemiacetals and Acetals The initial product of addition reactions of aldehydes and ketones with alcohols are known as hemiacetals. Compounds with both an -OH group and an -OR group bonded to the same carbon atom. Prentice Hall;Copyright 2007 2 • Ethanol forms hemiacetals with acetaldehyde and acetone. • Hemiacetals rapidly revert back to aldehydes or ketones by loss of alcohol and establish an equilibrium with the aldehyde or ketone. • When equilibrium is reached, very little hemiacetal is present. Prentice Hall;Copyright 2007 3 • A major exception occurs when the C=O and -OH functional groups that react are part of the same molecule. The resulting cyclic hemiacetal is more stable than a noncyclic hemiacetal. • Most simple sugars exist mainly in the cyclic hemiacetal form, as shown below for glucose, rather than in the open-chain form. Prentice Hall;Copyright 2007 4 • If a small amount of acid catalyst is added to the reaction of an alcohol with an aldehyde or ketone, the hemiacetal initially formed is converted into an acetal in a substitution reaction. • An acetal is a compound that has two -OR groups bonded to what was once the carbonyl carbon atom. Prentice Hall;Copyright 2007 5 The aldehyde or ketone from which an acetal is formed can be regenerated by reversing the reaction. Reversal requires an acid catalyst and a large quantity of water. Hydrolysis: A reaction in which a bond or bonds are broken and the -H and -OH of water add to the atoms of the broken bond or bonds. Prentice Hall;Copyright 2007 6 Chapter Summary • The carbonyl group is a C=O. The group is polar, with a partial (-) charge on O and a partial (+) charge on C. The O and the two substituents on the carbonyl-group C atom form a planar triangle. • The simplest aldehydes and ketones are known by common names. Aldehydes are named systematically by replacing the final -e in an alkane name with -al. • Ketones are named systematically by replacing the final -e in an alkane name with -one and numbering starting with 1 at the end nearer the group. The location of the carbonyl group is indicated by placing the number of its carbon before the name. Prentice Hall;Copyright 2007 7 Chapter Summary Cont. • Aldehyde and ketone molecules are polar, do not hydrogen-bond with each other, but can hydrogenbond with water. Small ones are water-soluble. Aldehydes and ketones are higher boiling than alkanes but lower boiling than alcohols. • Aldehydes and ketones are present in many plants, where they contribute distinctive, pleasant odors. Such natural aldehydes and ketones are widely used in perfumes and flavorings. • Formaldehyde (an irritating and toxic substance) is used in polymers, is present in smog-laden air, and is produced biochemically from ingested methanol. Prentice Hall;Copyright 2007 8 Chapter Summary Cont. • Acetone is a widely used solvent and is a by-product of food breakdown during diabetes and starvation. Many sugars are aldehydes or ketones. • Mild oxidizing agents (Tollens’ and Benedict’s reagents) convert aldehydes to carboxylic acids but have no effect on ketones. • With reducing agents, hydride ion adds to the C of the group in an aldehyde or ketone and hydrogen ion adds to the O to produce primary or secondary alcohols, respectively. Prentice Hall;Copyright 2007 9 Chapter Summary Cont. • Aldehydes and ketones establish equilibria with alcohols to form hemiacetals or acetals. • Hemiacetals, which have an -OH and an -OR on what was the carbonyl carbon, result from addition of one alcohol molecule to the C=O bond. • The more stable acetals, which have two -OR groups on what was the carbonyl carbon, form by addition of a second alcohol molecule to a hemiacetal. • The aldehyde or ketone can be regenerated from an acetal by treatment with an acid catalyst and a large quantity of water, which is an example of a hydrolysis reaction. Prentice Hall;Copyright 2007 10