Identification of an Unknown Carbonyl Compound
advertisement

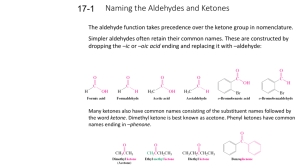
Identification of an Unknown Carbonyl Compound Chemistry 234 You will receive a liquid unknown that is either an aldehyde or ketone and your task is to determine what aldehyde or ketone it is. To aid in the identification of this unknown several tests can be performed. You should keep the unknown off of you because it can be a skin irritant. Below is a brief outline of the tests you can perform to aid you in the identification of the unknown. Details for the tests are on the back of this handout. The unknowns will come from the lists of Aldehydes and Ketones provided. 1. Determination of physical constants – set up a simple distillation and obtain the boiling point range for your unknown 2. Distinguishing an aldehyde from a ketone – Perform a CrO3 – H2SO4 test. In this test an aldehyde is oxidized by the reagent and there is a color change from orange to bluegreen. Ketones, on the other hand, are not oxidized and the color remains orange throughout the reaction. 3. Iodoform Test for Methyl Ketones – If you have a ketone, you can carry out the iodoform test to see if you have a methyl ketone. A methyl ketone is one that has a CH3 group directly attached to the carbonyl group (the C=O group) 4. Preparation of a 2,4-DNP derivative – One can react an aldehyde with 2,4dinitrophenylhydrazine to form a 2,4-dinitrophenylhydrazone derivative. These derivatives are solids and one can check the melting point of the derivative to the suspected carbonyl compound. 5. Spectroscopic analysis – Obtain an IR spectrum for your unknown and see if it matches the spectrum of the compound you think it is. In the IR room are a series of books called Sadtler Index which gives the IR spectra of several thousand organic compounds. In the Dupre Library reserve room (under CHEM 234) there is another IR book entitles Aldrich collection of IR that you may check as well. CrO3 – H2SO4 Test o Dissolve 3 drops of unknown in approximately 20 drops of acetone. Add 2-3drops of the H2Cr2O7 reagent and shake for one minute. A blue-green color and/or precipitate of that color is a positive test for aldehydes. (Color change may take a 2-3 minutes to occur) Iodoform Test o If the substance is water soluble, dissolve 2-3 drops of the unknown in 2 mL of water in a small test tube. Add 2 mL of 3M NaOH, and then slowly add 3mL of Iodine solution. In a positive test, the brown color disappears, and a yellow iodoform separates. o If the substance is water insoluble, dissolve it in 2mL of dioxane, and proceed as above. At the end dilute with 10mL water. o Iodoform is a yellow solid that melts at 119oC 2,4-DNP Test o Dissolve 10 drops (or 0.500g) of your carbonyl unknown in 20 mL of 95% ethanol. Add this solution to 15mL of the 2,4-DNP Reagent. Cool in an ice bath for 15 minutes. Collect the product by vacuum filtration and let dry for several days before obtaining a melting point.