Chapter 9 Study Questions
advertisement

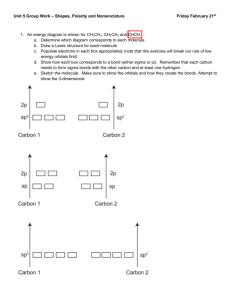
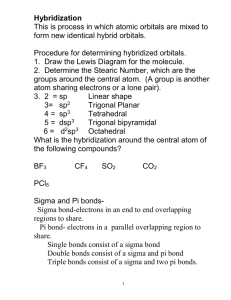
Olympic High School AP Chemistry Name ___________________________ Period 10 Bonding & Molecular Structure STUDY QUESTIONS Sections 10.1 – 10.2 1. What is the difference between a sigma(σ) and a pi(π) bond? 3. What are the approximate angles between regions of electron density in the following molecular geometries? Linear: Trigonal planar: Tetrahedral: 5. For each of the following molecular geometries, tell how many of each are used: # Electron Domains # Bonding Domains (shared pairs) # Non-bonding (unshared pairs) Tetrahedral: Linear: Bent: Trigonal-planar: Trigonal-pyramid: Octahedral: Trigonal-bipyramid: Square pyramid: See-saw: Valence Bond Theory 19. Draw the Lewis structure for the ClF2+ ion. a) What is its molecular geometry? b) What orbitals on Cl and F overlap to form bonds involving these elements? 21. Draw the Lewis structure for AlF4−. a) What is its molecular geometry? b) What orbitals on Al and F overlap to form bonds between these elements? 1 27. Draw the Lewis structure and then specify the molecular geometries for each of the following molecules or ions. Identify the hybridization of the central atom. XeOF4 BrF5 OSF4 Central Br in Br3− 45. Sketch the resonance structures for the nitrate ion, NO3−. Is the hybridization of the N atom the same or different in each structure? Describe the orbitals involved in bond formation by the central N atom. 65. Amphetamine is a stimulant. Replacing one H atom on the NH2, or amino, group with CH3 gives methamphetamine, a particularly dangerous drug commonly known as “speed”. (a) What are the hybrid orbitals used by the C atoms of the C6 ring, by the C atoms of the side chain, and by the N atoms? (b) Give approximate values for the bond angles A, B, and C. (c) How many σ bonds and π bonds are in the molecule? (d) Is the molecule polar or nonpolar? (e) Amphetamine reacts readily with a proton (H+) in aqueous solution. Where does this proton attach to the molecule? 2