REACTIONS OF ALDEHYDES AND KETONES
advertisement

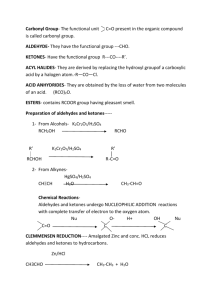
REACTIONS OF ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Things to Consider 1. Because the bond strength of the C=O bond (804 kJ/mol) is much stronger than the C=C bond (620 kJ/mol) the carbonyl group is less reactive than the C=C bond found in alkenes. 2. Because the carbonyl group is very polar, it does undergo many reactions but these are different from those that the alkenes undergo. 1. Combustion aldehyde or ketone + O2 CO2 + H2O Hydrogenation (a reduction reaction) 2. P t/1 0 1 M P a a ld e h y d e /k e to n e+ H 2 • a lc o h o l Aldehydes produce primary alcohols. H H HC C C H H O HH H O P t/1 0 1 M P a +H 2 H C C CH H H H H 1 p r o p a n o l p r o p a n a l • Ketones produce secondary alcohols HOH H H HO P t /1 0 1 M P a CC C H H+ H 2 H H p r o p a n o n e HC C C H HHH 2 p r o p a n o l 3. Oxidation (reaction with KMnO4 or H2Cr2O7) • ONLY ALDEHYDES ald eh y d e+w ater O C H K M n O 4 H C r2O 2 7 carb o x y licacid H K M n O 4 C H+H OHC 2 O 2r 2 7 H C C H+ 2 H + H e th a n o ica c id (a c e tica c id ) K M n O 4 H C C C H+H OHC 2 O 2r 2 7 H H p ro p a n o n e H H O e th a n a l HO H O N O R E A C T IO N Qualitative Tests for Aldehydes and Ketones 1. Fehling’s Solution (copper (II) solution) • This is an oxidation reaction in which the blue copper(II) solution will form an orange precipitate if there is a reaction. aldehydes + Cu2+ carboxylic acid + Cu (s) blue solution orange precipitate ketones + Cu2+ NO REACTION solution stays blue 2. Tollen’s Reagent (Silver Mirror Test) • • Silver ions in ammonia (NH3) Commercially used to “silver mirror” glass. H 3 + N a ld e h y d e+A g c o lo u rle s ss o lu tio n + ketone+A g N H 3 c a rb o x y lica c id+ A g (s ) b la c kp re c ip ita te N OR E A C T IO N solutionstayscolourless Reactions of Carboxylic Acids • Organic acids are “weak” acids, therefore they do not completely ionize in water. c a r b o x y lic a c id+ w a te r c a r b o x y la te io n + h y d r o n iu m io n O H O 2 C + H H C O 3 e th a n o ic a c id ( a c e tic a c id ) O + C + H O 3 H C O 3 e t h a n o a te io n ( a c e ta te io n ) 1. Neutralization c a rb o x y lica c id+ b a s e O +N a O H C H H C O 3 e th a n o ica c id ( a c e tica c id ) m e ta lc a rb o x y la te+w a te r O C + H O 2 + a H C ON 3 s o d iu m e th a n o a te ( s o d iu m a c e ta te ) 2. Test for Carboxylic Acids • Reaction with carbonate. c a r b o x y l i c a c i d + s o d i u m c a r b o n a t e s a l t( " o a t e " ) + c a r b o n d i o x i d e + w a t e r O O N a C O 2 3 ( a q ) C + H H C O 3 C + C O + H O 2 ( g ) 2 + N a H CO 3 s o d i u m e t h a n o a t e ( s o d i u m a c e t a t e ) e t h a n o i c a c i d ( a c e t i c a c i d ) 3. Formation of an Ester (reaction with an alcohol) H S O / 2 4 c a r b o x y l i c a c i d + a l c o h o l HO H m e t h a n o l HO H O H O CH+ HCC 2 H S O / 2 4 C H HCC + H O H O H H e t h a n o i c a c i d ( a c e t i c a c i d ) e s t e r+ w a t e r H H m e t h y l e t h a n o a t e ( m e t h y l a c e t a t e )