NAME__________________________________ 9/14 rev ws for
advertisement

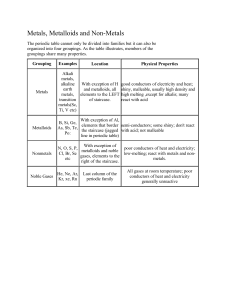
NAME__________________________________ 12/4 rev ws for exam #1 1] What are the six most abundant elements in the human body? Calcium, nitrogen, carbon, hydrogen, oxygen, phosphorus [CaNCHOP] 2] What are the four most abundant elements in the earth’s crust? Iron, aluminum, silicon, oxygen [FeAlSiO] 3] What does the Law of Conservation of Matter say?matter is not created or destroyed in a chemical change 4] Who discovered the Law of Conservation of Matter? Lavoissier 5] In 1804, who determined that the Laws of Definite and Multiple Proportion and Law of Conservation of Matter told us that matter is atomic? Dalton 6] Who discovered the electron? Thomson 7] Who discovered the proton and nucleus? Rutherford 8] Who first put elements in the periodic table based on their chemical properties? Mendeleev 9] What are the seven diatomic molecules? Hydrogen, oxygen, nitrogen, fluorine, iodine, chlorine, bromine [HONFIClBr] 10] Why are these seven molecules diatomic? They bond to themselves when they are not bonded to other atoms – They form X2 molecules in the free state. 11] How many oxygen atoms are in: a] Ca(NO3)2 6 b] 3H2O 3 c] MgSO4∙7H2O 11 d] 3Ga2(SO4)3 36 12] Changing the number of protons in the nucleus changes the: atom 13] Changing the number of neutrons in the nucleus changes the: isotope (mass number) 14] Changing the number of electrons changes the: ion/charge/oxidation number 15] a] On the periodic table, where are the metals? Left of the staircase b] Where are the non-metals? Right of the staircase c] Where are the metalloids? Along the edge of the staircase 16] Identify the metalloids: Si, Ge, As, Sb, Te, Po, At 17] If Atom Q has 4 isotopes, 86, 88, 89 and 90 with percent abundances of 0.04%, 0.16%, 5.90 % and 93.90% respectively, what is it’s atomic mass (round to the hundredths)? 89.94 amu 18] a] What are periods on the periodic table? Elements in the same row b] What are groups on the periodic table? Elements in the same column 19] a] What are alkali metals? 1A b] What are alkali-earth metals? 2A c] What the halogens? 7A d] What are the noble gases? 8A e] What are the transition metals? Between the 2A’s and 3A’s f] What are the rare-earth metals? Bottom of the periodic table 20] What is the preferred ion for: a] Ga +3 b] Ba +2 c] P -3 d] F -1 e] O-2 21] Predict the formula for: a] aluminum and iodine AlI3 b] calcium and chlorine CaCl2 c] barium and nitrogen Ba3N2 d] magnesium and oxygen MgO e] sodium and phosphorus Na3P 22] What do the A and Z values represent? A = mass number and isotope – number of protons + neutrons; Z = atomic number – number of protons 23] Complete the table below: complete the following chart: element number of protons number of neutrons 19 -1 F 9 10 f] K +1 number of electrons 10 9 57 Fe+2 26 65 26 29 +2 ___Cu____ 29 75 31 -3 As 36 33 24 27 42 36 33 82 210 128 78 +4 Pb 82 115 In 49 49 235 66 92 143 86 138 51 88 +4 U 92 224 Rn 86 0 86