The Periodic Table
advertisement
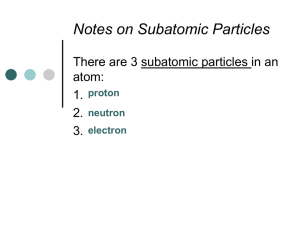
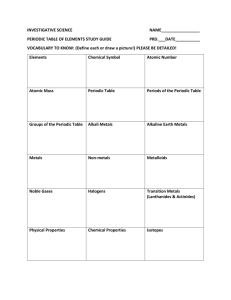
2.2 The Periodic Table The Periodic Table Every block represents an element Every periodic table has a key The Periodic Table Where did the modern periodic table come from? Newlands Mendeleyev Modern Periodic Table Period or Series – a horizontal row of elements, numbered 1-7) Group or Family – a vertical row of elements (numbered 1-18 on the modern table) (long ago: roman numerals) Modern Periodic Table Staircase – divides the metals from the nonmetals Metalloids – look like metals but act like nonmetals The Periodic Law States that when elements are arranged in order of increasing atomic mass, certain physical and chemical characteristics repeat in a regular pattern (periodically) Modern Periodic Table Atomic Mass – defined relative to the mass of a carbon atom, which is given a mass of 12 amu AMU (atomic mass unit) – defined as 1/12 the mass of a carbon atom Period / Group Names Group 1 – Alkali Metals Soft, silver colored metals that react violently with water React with the halogens Group 2 – Alkaline Earth Metals Light, reactive metals that form oxide coatings with air exposure (ex MgO, CaO) Period / Group Names Group 17 – Halogens Extremely reactive (F is the most) Form hydrogen halides (ex HCl) Group 18 – Noble Gases Extremely low chemical reactivity Period / Group Names Transition Elements Groups 3-12 Representative Elements Groups 1-2, & 13-18 * Hydrogen is the exception to almost every rule in chemistry The Atom The Atom Is the smallest part of an element that is still representative of that element A neutral particle composed of a nucleus (containing protons and neutrons) and electrons # electrons = # protons Electrons eA small, negatively charged subatomic particle Has negligible mass Nucleus The centre of an atom Contains most of the mass and all of the positive charge of an atom Protons p+ Positively charged subatomic particle Found in the nucleus Mass of 1 amu Neutrons n Uncharged (neutral) Found in the nucleus Mass of 1 amu Atomic Number and Atomic Mass Atomic Number The number of protons an atom has Different for each element Atomic Mass (Mass Number) # protons + # neutrons