The halogens
advertisement

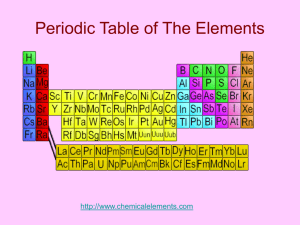
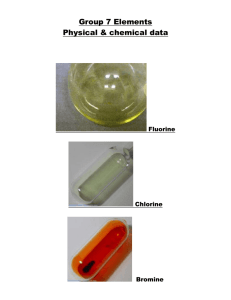
The halogens The halogens: Fluorine Chlorine Bromine Iodine The halogens are found on the second column from the right on the periodic table. They contain seven valence electrons. Their characteristics include having a bad smell and harming the skin and mucous membranes. All of the halogens except iodine are poisonous. The halogens exist as diatomic molecules and in room temperature they are found in each of the different states of matter, Fluorine having the lowest melting and boiling points and Iodine having the highest ones. All halogens are so reactive that they can only be found in nature bonded to another substance. Halogens exist as diatomic molecules in room temperature. The reactiveness of the halogens becomes lower going down the periodic table. Going down the periodic table, the halogens get denser. Often, Halogens bond with elements from group one on the periodic table. They are all soluble in water. Uses of halogens Fluorine: Fluorine is the most reactive of the halogens. Alone it is poisonous, but in compounds, its characteristics are completely different. Many types of toothpaste contain fluorine to prevent tooth decay, and some water supplies have chloride added to them for the same reason. Fluorine is also commonly used bonded to Carbon, as Teflon. Chlorine: Chlorine has many more uses than Fluorine. Chlorine is most commonly found bonded to sodium, as sodium chloride, or more commonly known as salt. Chloride is also used to kill bacteria in swimming pools and drinking water. It is used to bleach paper and in disinfectants. Chloride is also found in many pesticides and weed killers. Bromine: Bromine is also used in pesticides. It is also a compound in some medicines, and silver bromine is used in photographic film. Iodine: Iodine is the least reactive of the halogens. Because it is an antiseptic, it is mainly found in disinfectants, diluted with alcohol.