Group 7 Halogens: Properties & Uses Data Sheet
advertisement

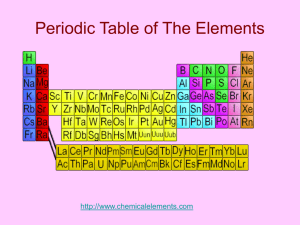
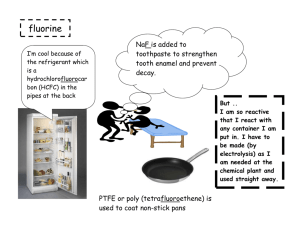
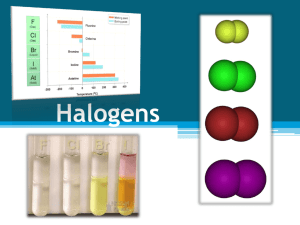
Group 7 Elements Physical & chemical data Fluorine Chlorine Bromine Iodine Astatine Selected Properties of the Group 7 Halogens Symbol Atomic Electron and Number arrangement Name State and colour at room temperature, colour of vapour when heated Melting point Boiling atom point radius nm F Fluorine 9 2.7 pale yellow gas -220oC, 53K -188oC 85K 0.072 Cl Chlorine 17 2.8.7 green gas -102oC, 173K -34oC, 239K 0.099 Br Bromine 35 2.8.18.7 dark red liquid, brown vapour -7oC, 266K 59oC, 332K 0.114 I Iodine 53 2.8.18.18.7 dark crumbly solid, purple vapour 114oC, 387K 184oC, 457K 0.133 At Astatine 85 2.8.18.32.18.7 302oC 575K 380oC 653K 0.140 black solid, dark vapour electron configuration fluorine chlorine bromine iodine astatine F Cl Br I At [He]2s22p5 [Ne]3s23p5 [Ar]3d104s2 4p5 [Kr]4d105s2 5p5 [Xe]4f14 5d106s2 6p5 Occurrence and Extraction The halogens are too reactive to occur free in nature. Fluorine is mined as fluorspar, calcium fluoride and cryolite. It is extracted by electrolysis as no oxidant will oxidise fluorides to fluorine. Chlorine is also found in minerals such as rock-salt, and huge quantities of chloride ions occur in seawater, inland lakes and subterranean brine wells. It is obtained by the electrolysis of molten sodium chloride or brine. Bromine is also found as the bromide ion in seawater, and in larger quantities in brine wells, from which it is extracted. Iodine is mined as sodium iodate(V), NaIO 3, which is present in Chile saltpetre. It is obtained by reaction with sodium hydrogensulfite. Electron Affinity (M-M-)kJ mol-1 F Cl Br I -333 -348 -324 -295 Ionisation Energies/kJ mol-1 F Cl Br I 1st 1681 1251.1 1139.9 1008.4 2nd 3374 2297 2104 1845.9 3rd 6050 3826 3500 3200 4th 8408 5158 4560 4100 The Halogens in their Elemental Form Fluorine (F2), a highly toxic, colorless gas, is the most reactive element known so reactive that asbestos, water, and silicon burst into flame in its presence. It is so reactive it even forms compounds with Kr, Xe, and Rn, elements that were once thought to be inert. Fluorine is such a powerful oxidizing agent that it can coax other elements into unusually high oxidation numbers, as in AgF2, PtF6, and IF7. Fluorine is so reactive that it is difficult to find a container in which it can be stored. F2 attacks both glass and quartz, for example, and causes most metals to burst into flame. Fluorine is handled in equipment built out of certain alloys of copper and nickel. It still reacts with these alloys, but it forms a layer of a fluoride on the surface that protects the metal from further reaction. Fluorine is used in the manufacture of Teflon or poly(tetrafluoroethylene), (C2F4)n which is used for everything from linings for pots and pans to gaskets that are inert to chemical reactions. Large amounts of fluorine are also consumed each year to make the freons (such as CCl2F2) used in refrigerators. Chlorine (Cl2) is a highly toxic gas with a pale yellow-green color. Chlorine is a very strong oxidizing agent, which is used commercially as a bleaching agent and as a disinfectant. It is strong enough to oxidize the dyes that give wood pulp its yellow or brown color, for example, thereby bleaching out this color, and strong enough to destroy bacteria and thereby act as a germicide. Large quantities of chlorine are used each year to make solvents such as carbon tetrachloride (CCl4), chloroform (CHCl3), dichloroethylene (C2H2Cl2), and trichloroethylene (C2HCl3). Bromine (Br2) is a reddish-orange liquid with an unpleasant, choking odor. The name of the element, in fact, comes from the Greek stem bromos, "stench." Bromine is used to prepare flame retardants, fireextinguishing agents, sedatives, antiknock agents for gasoline, and insecticides. Iodine is an intensely colored solid with an almost metallic luster. This solid is relatively volatile, and it sublimes when heated to form a violet-colored gas. Iodine has been used for many years as a disinfectant in "tincture of iodine." Iodine compounds are used as catalysts, drugs, and dyes. Silver iodide (AgI) plays an important role in the photographic process and in attempts to make rain by seeding clouds. Iodide is also added to salt to protect against goiter, an iodine deficiency disease characterized by a swelling of the thyroid gland. Some of the chemical and physical properties of the halogens are summarized in the table below. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the melting point, boiling point, intensity of the color of the halogen, the radius of the corresponding halide ion, and the density of the element. On the other hand, there is a regular decrease in the first ionization energy as we go down this column. As a result, there is a regular decrease in the oxidizing strength of the halogens from fluorine to iodine. F2 > Cl2 > Br2 > I2 oxidizing strength Halogens Halogen Fluorine Compounds fluorides Teflon (fluorocarbon) Freon (fluorocarbon) Chlorine Bromine Iodine Properties protect teeth against decay heat-resistant plastic Uses drinking water and toothpaste non-stick pans and electrical insulation refrigerant hypochlorous acid and hypochlorites easily liquefied gas with high heat of vaporization powerful oxidizing agent, germicide, bleaching agent polyvinyl chloride (PVC) tough plastic silver bromide light sensitive bromides of sodium and potassium silver iodide, iodides of sodium and potassium sedative purify water for drinking, pools, sewage treatment, bleaching industry, household bleaches and disinfectants covering for furniture and floors photographic film, plates, paper headache powders light sensitive, prevent goiter photography, make ‘iodized’ table salt ………………..and there’s more at …………………………….… www.chemtopics.com/elements/halogen/halogen.htm www.theodoregray.com/PeriodicTable/Elements/Halogens/index.s7.html