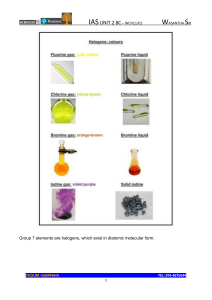
Halogens By : Pranit Vijay What Are Halogens ? Halogens is the seventh group in the periodic table and the word halogens means salt formers and all the elements in this group forms salts readily. Properties Of Halogens Colour gets darker. Restiveness reduces. Density, melting and boiling point increases. They are smelly and poisonous. They are non metals and are bad conductors. ● Fluorine is the most reactive halogen in the halogen group. ● ● ● ● ● Uses Of Fluorine, Chlorine Bromine Fluorine ● Protects teeth against decay with tthe process called demineralization. ● It is used in CFC’s which is a key component in AC’s and refrigerators. ● It is added to water to increase fluoride content. Chlorine ● Found in combination with sodium as rock salt. ● Used as a disinfectant in pools and water supplies. ● Used in consumer products like : paper, paint, etc. Bromine ● Used on camera films. ● Found in fire retardants. ● Used used in tranquilizers and sedatives. 🙏THANK YOU🙏 .