Intro to chemistry notes

Intro to chemistry notes
What is Chemistry?
Chemistry is the study of the ____composition______________ and _______properties___________ of
____matter_________ and the _______changes___________ it undergoes.
What’s Matter?
Matter is anything that has _______mass__________ and takes up ______space________.
Mass is the amount of _____matter_________ that a substance possesses.
Weight is the effect of ___gravity__________ on that mass.
Mass vs. Weight
Say you weigh ___150_______ pounds on Earth and you travel to the moon. You will only weigh
__25______ pounds on the moon, because the moon has _1/6______ the ___gravity_________. Your
____weight_________ changes, but you still have the same _____mass__________ and take up the same amount of _____space__________.
Types of Matter
Matter is made of either __________pure substances___________ or _____mixtures__________.
A pure substance is made of only ____one______ type of particle.
A mixture is made of ___two ______ or ____more__________ types of particles.
Pure Substances
A pure substance can be _____subdivided_____________ into smaller and smaller particles, but every piece still has the same ______properties_____________ as the whole. A pure substance that is made of only one type of __atom___________ is called an ______element_________.
Atoms & Elements
Atoms are considered the ____building____________ blocks of all _____matter_________, and cannot be ___created____________ or _________destroyed_______. There are approximately _____109_____ different types of atoms, each with its own unique _____composition___________.
If two or more atoms are _______joined__________ together, this substance is called a
_____compound_______________.
element compound
Elements
Compounds
Compounds are made by forming chemical ___bonds________ between ____atoms______. The smallest naturally-occurring unit of a ____compound____________ is called a ________molecule_________.
If substances are combined but they do __not_________ form chemical ____bonds__________, the substance is called a ______mixture__________.
Mixtures
A mixture that has the same _____composition_____________ throughout the substance is called a
_______homogeneous________________ mixture.
Examples of homogeneous mixtures:
Salt water
Vanilla pudding Air
Mayonnaise
A mixture that does not have the same _____composition____________ throughout the substance is called a _______heterogeneous___________ mixture.
Examples of heterogeneous mixtures:
Rocky Road ice cream
Mixed nuts soil
Fruit salad
Flowchart of Matter
Matter
Pure Substances Mixtures
Compound
Hetero- geneous
Homo- geneous
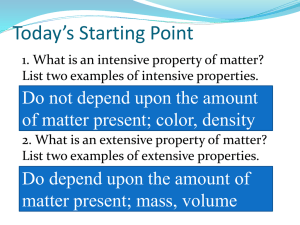
Properties of Matter
____Physical_____________ properties are those characteristics that can be ____observed___________ using our senses without changing the ____identity______ of the substance. Common physical properties include:
color density
Boiling point odor Melting point Physical state
____Chemical________ properties describe a substance’s ability to undergo ____changes_______ in
____identity______.
Examples: ____Iron________ reacts with _____air______ and ____water_______ to form
______rust_____
____Flour___________, ___egg_______ and _____sugar_________ become _____cake________ in the presence of ______heat_____.
Changes in Matter
The changes that matter undergoes can be classified as either _____physical_______ or
____chemical________ changes.
Physical changes alter the _____physical_________ properties of the substance, but not its basic
_______composition________________. *** A physical change: changes what you ___look______ like, but not ___who______ you ____are_______.***
A chemical change alters the ____composition__________ and _________structure______ of a substance.
The ____products_______ of a chemical change have ____different_______ properties! Chemical changes are always accompanied by changes in ___energy____________. *** A chemical change: changes ____everything___________ about you!***
Signs of a Chemical change
Change in _____color________ or _____odor________
Formation of a _____gas_____
Formation of a ___precipitate__________ (solid)
Change in ______light_______ or ____heat_______
Qualitative vs. Quantitative
Qualitative deals with the ___qualities________ of a substance. **These can be ___observed______ without regard for the ____amount________ of the substance.
Examples: ____color_________, ____odor_______, _____state of matter_______
Quantitative analysis deals with ____measuring__________ the substance.
Examples: _____mass_______, _____volume__________, _____temperature_______
THINK! Quantitative = _____QUANTITY____________!