File
advertisement

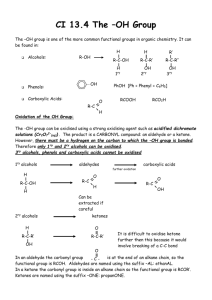
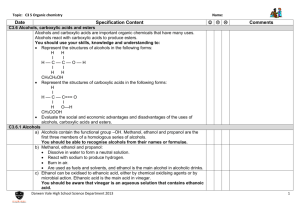
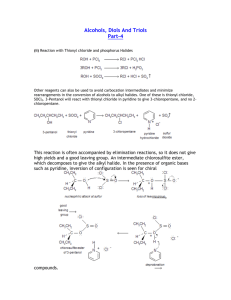
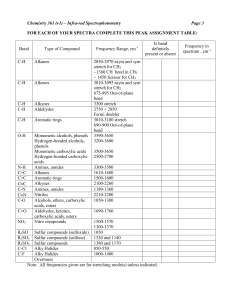
Alcohol & Phenol Reactions Alcohol Reactions • 1. Dehydration - elimination of water • water is eliminated from adjacent carbon atoms and a second bond is formed between the 2 carbon atoms • uses a large amount of alcohol and a small amount of acid (H2SO4) as the catalyst • order of reactivity: – tertiary > secondary > primary Dehydration Examples 1. Dehydrate butanol. 2. Dehydrate 2-butanol. 3. Dehydrate 2-methyl-2-butanol. • 2. Halide Substitution - alcohols react with concentrated hydrohalogens (HCl, HBr, HF) to be converted into alkyl halides • competes with the elimination reaction (dehydration) but this reaction is favored using a large excess of concentrated acid (HCl and zinc chloride) • we can distinguish between 1°, 2° and 3° alcohols by their different rates of reaction using the Lucas Test • order of reactivity: – tertiary > secondary > primary Halide Substitution Examples: The Lucas Test • 1. Primary alcohols - are not converted to alkyl chlorides at room temp; dissolve completely • 2. Secondary alcohols – dissolves, but within 5 minutes, the alkyl chloride separates out • 3. Tertiary alcohols – react immediately; the insoluble tertiary alkyl chloride separates out as a cloudy layer • 3. Esterification – alcohols react with carboxylic acids to form esters and water • esters – compounds in which the proton of an acid is replaced by an organic group • General formula is • Esters are named as derivatives of BOTH the carboxylic acid and the alcohol – The 1st word denotes the alcohol group (R) and is named like a hydrocarbon with a –yl ending – The 2nd word is derived from the name of the parent acid, with the “-ic acid” ending changed to an “-ate” ending. Esterification Examples • Ex.1 React methanol with acetic acid. • Ex.2 React propanol with butanoic acid. • Ex.3 React 2-propanol with butanoic acid. • 4. Oxidation - the most important method of preparing aldehydes, ketones and carboxylic acids from alcohols aldehyde ketone carboxylic acid • Uses Jones Reagent in what is called the Bordwell-Wellman Test • Jones Reagent – made by dissolving CrO3 in water with H2SO4 to give chromic acid, which is orange in color Oxidation Examples • Primary alcohols - oxidized in a 2-step process to first give an aldehyde, and then an acid • Ex.; Oxidize ethanol. • Secondary alcohols - oxidized to form ketones • Ex.; Oxidize 2-butanol. • Tertiary alcohols - have no C-H bonds on the hydroxyl carbon, therefore they cannot undergo oxidation. • Ex.; Oxidize tert-butanol. Reactions of Phenols • Phenols DO NOT undergo dehydration or substitution reactions because it is very hard to break the C-O bond when the carbon atom is part of an aromatic ring • They will form esters because the O-H bond is breaking, not the C-O bond • 1. Esterification - phenol reacts with an acid anhydride (two acid molecules with a water removed) to form a carboxylic acid and an ester • Ex.; part a: form acetic anhydride part b: react phenol with acetic anhydride • 2. Oxidative Coupling - uses potassium ferricyanide, K3Fe(CN)6, as the catalyst • -three types of coupling: ortho-ortho, ortho-para, and para-para • Ex. React phenol with K3Fe(CN)6. • Ex. React p-methylphenol with K3Fe(CN)6.