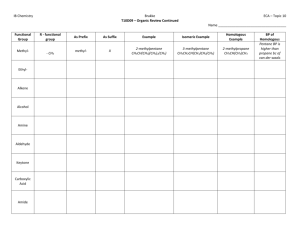
word - Seattle Central College
advertisement
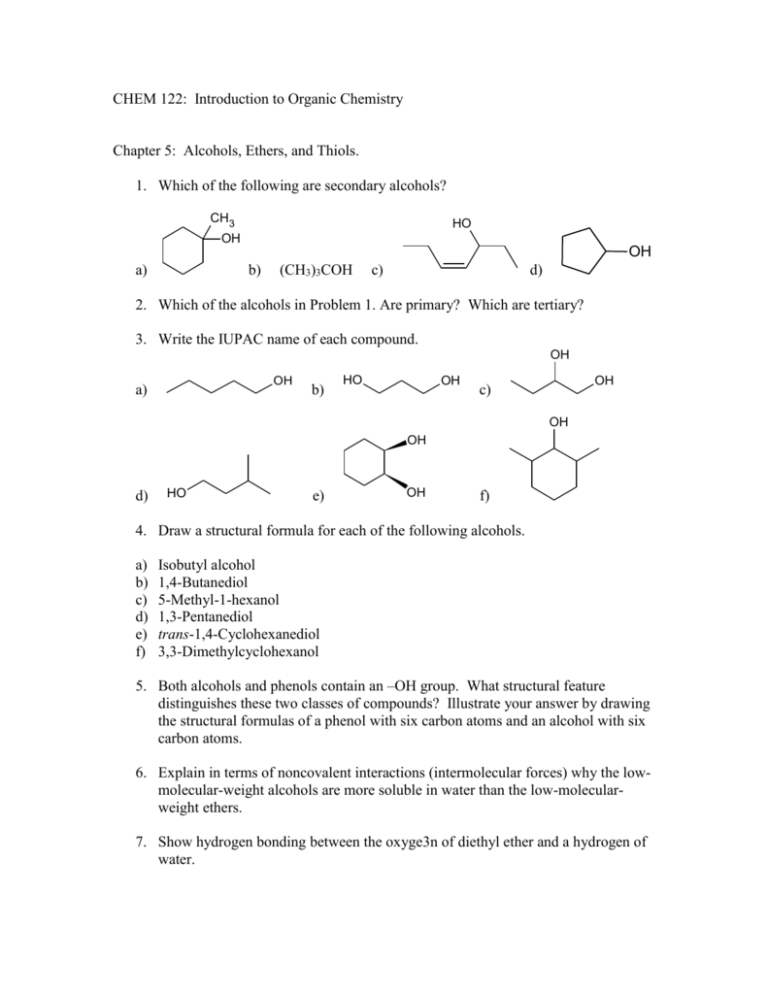
CHEM 122: Introduction to Organic Chemistry Chapter 5: Alcohols, Ethers, and Thiols. 1. Which of the following are secondary alcohols? CH3 HO OH OH a) b) (CH3)3COH c) d) 2. Which of the alcohols in Problem 1. Are primary? Which are tertiary? 3. Write the IUPAC name of each compound. OH OH a) b) HO OH OH c) OH OH d) HO e) OH f) 4. Draw a structural formula for each of the following alcohols. a) b) c) d) e) f) Isobutyl alcohol 1,4-Butanediol 5-Methyl-1-hexanol 1,3-Pentanediol trans-1,4-Cyclohexanediol 3,3-Dimethylcyclohexanol 5. Both alcohols and phenols contain an –OH group. What structural feature distinguishes these two classes of compounds? Illustrate your answer by drawing the structural formulas of a phenol with six carbon atoms and an alcohol with six carbon atoms. 6. Explain in terms of noncovalent interactions (intermolecular forces) why the lowmolecular-weight alcohols are more soluble in water than the low-molecularweight ethers. 7. Show hydrogen bonding between the oxyge3n of diethyl ether and a hydrogen of water. 8. Arrange these compounds in order of increasing boiling point. Values in oC are -42, 78, 117, and 198. a) b) c) d) CH3CH2CH2CH2OH CH3CH2OH HOCH2CH2OH CH3CH2CH3 9. From each pair, select the compound that is more soluble in water. a) CH3OH or CH3OCH3 OH CH2 b) CH3CHCH3 or c) CH3CH2CH2SH CH3CCH3 or CH3CH2CH2OH 10. Give the structural formula of an alkene or alkenes from which each alcohol can be prepared. a) b) c) d) e) 2-Butanol 1-Methylcyclohexanol 3-Hexanol 2-Methyl-2-pentanol Cyclopentanol 11. Both 2,6-diisopropylcyclohexanol and the intravenous anesthetic Propofol are insoluble in water. Show how these two compounds can be distinguished by their reaction with aqueous sodium hydroxide. OH 2,6-Diisopropylcyclohexanol OH 2,6-Diisopropylphenol (Propofol) 12. Write equations for the reaction of 2-butanol with these reagents. a) H2SO4, heat b) K2Cr2O7, H2SO4 13. Show how to convert cyclohexanol to these compounds. a) b) c) d) Cyclohexene Cyclohexane Cyclohexanone Bromocyclohexane 14. Show reagents and experimental conditions to synthesize each compound from 1propanol. Br Br Br (e) (d) (f) OH (b) O (c) (a) OH (g) OH O (i) (h) O H 15. Write the common name for each ether. O a) b) [CH3(CH2)4]2O c) CH3CHOCHCH3 16. Write the IUPAC name of each thiol. SH a) CH3CH2CHCH3 b) CH3CH2CH2CH2SH c) 17. Write the common name for each thiol in Problem 16. 18. Explain why methanethiol, CH3SH, has a lower boiling point (6oC) than methanol, CH3OH (65oC), even though methanethiol has a higher molecular weight. 19. Draw structural formulas and write IUPAC names for the eight isomeric alcohols with the molecular formula C5H12O. 20. Draw structural formulas and write common names for the six isomeric ethers with the molecular formula C5H12O. 21. 1,4-Butanediol, hexane, and 1-pentanol have similar molecular weights. Their boiling points, arranged from lowest to highest, are 69oC, 138oC, and 230oC. Which compound has which boiling point? 22. Of the three compounds given in Problem 21., one in insoluble in water, another has a solubility of 2.3 g/100g water, and one is infinitely soluble in water. Which compound has which solubility? 23. Show how to prepare each compound from 2-methylcyclohexanol. O a) CH3 b) CH3 d) CH3 OH c) CH3 24. The mechanism of the acid-catalyzed dehydration of an alcohol to an alkene is the reverse of the acid-catalyzed hydration of an alkene. The dehydration mechanism occurs by the following three steps: Step 1: Add a proton. Step 2: Break a bond to form stable molecules or ion. Step 3: Take away a proton. These three steps are illustrated here by the dehydration of 2-butanol to give 2-butene. Use curved arrows to show the flow of electrons in each step; that is, show how each bondmaking or bond-breaking step occurs. Step 1: OH H+ H O H H O H Step 2: + H2O Step 3: + H+ and H 25. Use the reactions we learned in Chapter 3 and this chapter to show how to bring about the following chemical transformations. Some transformations will require only one step. Others will require two or more steps. OH a) HO b) O c) O d) O CH 2OH OH e) CH3 f) OH