Organic chemistry and Biological chemistry for Health Sciences
advertisement

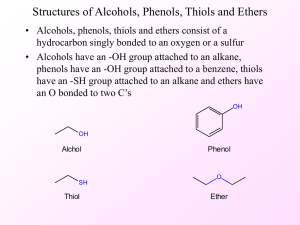
Organic chemistry and Biological chemistry for Health Sciences 59-191 Lecture 9 ALCOHOLS: When OH group is covalently attached to a saturated carbon atom in a molecule it is called an alcohol. Monohydric alcohols are those with one OH group per molecule and no other functional group. When the molecule has two OH groups, it is called dihydric alcohol. The molecules of a trihydric alcohol have three alcohol groups. An alcohol can be classified as primary, secondary or tertiary according to the condition of the carbon that holds the OH group. When held by a primary carbon, one that has only one R group directly joined to it, the alcohol is a primary alcohol. In a secondary alcohol, the -OH group is held by a 20carbon. When the OH group is joined to a 30 carbon, the alcohol is a tertiary alcohol. Simple alcohols have common names devised by writing the word alcohol after the name of the alkyl group holding the OH group IUPAC rules to name an alcohol: Determine the parent alcohol by selecting the longest chain of carbons that includes the carbon atom to which the OH group is attached. Name the parent alcohol by changing the name ending of the alkane that corresponds to this chain from –e to –ol. Number the parent chain from whichever end gives the lower number to the carbon holding the OH group. The location of the OH group thus take precedence over the location of alkyl groups (or halogen atoms). Write the number that locates the OH group in front of the name of the parent and separate this number from the parent’s name by a hyphen. Next, alphabetically prefix the names of the substituents and their location numbers. Use commas and hyphens in the usual way. When two or more OH groups are present, use name endings such as –diol (for two OH groups), -triol, and so forth. Immediately in front of the name of the parent portion of the alcohol, write the two, three, or more numbers that show the locations of the OH groups. If no parent alcohol name is possible or convenient, then the OH group can be treated as substituent named hydroxy. Physical properties of alcohols: Alcohols have much higher boiling points and much greater solubilities in water than alkanes of the same formula mass because their OH group can both donate and accept hydrogen bonds. It is also quite polar. The partial positive charge on H in the OH group and the partial negative charge on the O of another OH group forms hydrogen bond. An individual alcohol molecule is attracted to two neighbouring molecules of alcohol by two hydrogen bonds. Water molecules have three hydrogen bonds between them. So water, despite having a lower formula mass than methyl alcohol, has a higher boiling point. CHEMICAL PROPERTIES OF ALCOHOLS: The loss of water (dehydration) and the loss of hydrogen are two important reactions of alcohols. In the presence of heat and a strong catalyst an alcohol is dehydrated to an alkene. A water molecule splits out and a carbon-carbon double bond emerges. The pieces of the water molecule, one H and one OH, come from adjacent carbons. When it is possible for two alkenes to be made, the more highly branched alkene forms. This is the alkene with the greater number of alkyl groups attached to the double bond. The more branched alkene predominates because it is more stable. In an organic compound oxidation can be defined by loss of 2H or the gain of O by a molecule. A reduction is the loss O or gain of 2H by a molecule. The oxidation of an alcohol is an example of the loss of 2H, which is why the reaction is often called dehydrogenation rather than oxidation. Enzymes that catalyze such reactions are called dehydrogenases. Only primary and secondary alcohols can be oxidized by the loss of 2H atoms. Molecules of tertiary alcohols do not have an H atom on the carbon that holds the OH group, so 30 alcohols cannot be oxidized by dehydrogenation. Oxidation of 10 and 20 alcohol gives a product with carbon-oxygen double bond. 10 alcohol is oxidized first to an aldehyde, but because aldehydes are more easily oxidized than 10 alcohols excess oxidizing oxidize the aldehyde further to carboxylic acids. The problem of halting oxidation of the alcohol at the aldehyde stage does not occur in body cells, because different enzyme is required for each oxidation step. Strong oxidizing agents like MnO4- or Cr2O72- oxidize secondary alcohols to ketones. Dehydrogenases accomplish the identical overall reaction in vivo. PHENOLS: Phenols are molecules with at least one OH group directly attached to a benzene ring. The simplest member of this family is also called phenol, and it is a raw material to make aspirin. Phenols are very weak acids. But they are in general strong enough to neutralize OH -. Phenol is able to donate H to OH- because the anion of phenol is more stable than the anion from alcohol. Oxidizing agents, like permanganate or dichromate easily oxidizes phenol rings. But they give complex mixtures of highly coloured products. In vivo special enzymes can oxidize certain phonol rings. Benzene ring in phenol is more readily oxidized because the ring is electron rich. Some of the electron density associated with the two unshared electron pairs on the oxygen in phenol spreads out into the space occupied by the six of the electrons of the benzene ring. Since oxidizing agents are electron seekers the increased electron density at the ring exposes it to oxidative attack. Unlike alcohol phenols cannot be dehydrated. ETHERS: Ethers are compounds whose molecules have two organic groups joined to the same oxygen atom, R-O-R/. A carbon atom joined to the bridging oxygen cannot be the carbon of a carbonyl group, C=O. In the common names of ethers, the names of groups attached to O are placed before the word ether. Ethers do not react with strong acids, with bases, or with strong oxidizing agents or reducing agents. So they are chemically stable. Because ether cannot donate hydrogen bonds boiling point of simple ethers is more like to those of alkanes of comparable formula masses than those of the alcohols. Since ethers can accept hydrogen bonds, they are more soluble in water than alkanes. In low temperature ether is formed if alcohol is heated in the presence of an acid catalyst.