Exam #3 Review Sheet CHEM 2124 Spring 2014
advertisement

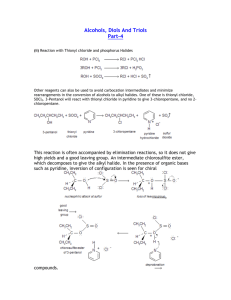
Exam #3 Review Sheet CHEM 2124 Spring 2014 Chapter 13 – Unsaturated Hydrocarbons Definitions Unsaturated Hydrocarbons Alkenes Alkynes Aromatic Hydrocarbons Consitutional Isomers Stereoisomers Cis/trans isomers Addition Reactions Hydrogenation Halogenation Hydrohalogenation Hydration Resonance structures Resonance hybrid Substitution Reactions Basic Concepts General molecular formula for alkenes (CnH2n) and alkynes (CnH2n-2) Molecule shapes and bond angles for alkenes, alkynes, and aromatics Why do alkenes show cis/trans isomerism? Do all alkenes show cis/trans isomerism? What is the difference between a cis isomer and a trans isomer? What are the products of the following alkene reactions: hydrogenation, halogenations, hydrohaolgenation, hydration? What catalysts are required for any of the above four mentioned alkene reactions? Markovnikov’s Rule: applies to the addition of a hydrohalogen (HCl) or water (H-OH) to an asymmetrical alkene. “The rich get richer” – What are the structures of both major and minor predicted products in these types of reactions? What are the products of the reaction between an aromatic ring compound and chlorine (Cl2)? What are the products of the reaction between an aromatic ring compound and nitric acid (HNO3)? What catalysts are needed to drive the two aromatic ring compound reactions? Nomenclature Alkene suffix = -ene Alkyne suffix = -yne The longest carbon chain that contains the functional group is the parent chain Number the parent chain so the functional group is given the lowest possible number Then number and name subsituents – all previous rules about alphabetizing, etc. remain the same Include the cis/trans designation when required Monosubstituted aromatic rings: know the structures of toluene, phenol, and aniline Disubstituted aromatic rings: o know the o-, m-, p- designation o know the structures of o-xylene, m-xylene, p-xylene o use common name roots when possible (ex: 2-methyl phenol) – the position of the functional group on the ring is automatically position #1 and the number “1” is omitted from the name o Number to give the lowest possible numbers around the ring to substituents Polysubstituted aromatic rings: same rules apply as to disubstituted rings – do not use o- m- p- designations here Recommended Textbook Practice Problems (All answers are in the back of the book): 13.31, 13.35, 13.39, 13.41, 13.43, 13.45, 13.51, 13.53, 13.55, 13.57, 13.59, 13.61, 13.63, 13.65, 13.73, 13.77, 13.79, 13.81, 13.83 (a and b only), 13.85 Chapter 14 – Organic Compounds with Oxygen and Halogens Definitions Alcohols Ethers Alkyl Halides Primary Alcohols Secondary Alcohols Tertiary Alcohols Intermolecular hydrogen bonding Dehydration Reactions Oxidation Reactions Heteroatoms Basic Concepts Draw and describe a primary, secondary, and tertiary alcohol Alcohols are “bent” in shape and tetrahedral about the oxygen atom Alcohols hydrogen bond with one another and thus have higher boiling and melting points that similar molecular weight hydrocarbons The most important physical property of an alcohol is the polarity of the –OH group Low molecular weight alcohols will dissolve in polar solvents (water) – high molecular weight alcohols will dissolve in organic solvents but will not be water soluble Loss of water from an alcohol is known as dehydration (an elimination reaction). Does this require a catalyst? Zaitsev’s Rule: The major product in al elimination reaction is the one that has more alkyl groups directly attached to the newly formed C=C bond Primary alcohols oxidize into aldehydes which then further oxidize into carboxylic acids Secondary alcohols oxidize into ketones Tertiary alcohols do not oxidize Ethers are “bent” in shape and tetrahedral about the oxygen atom just like alcohols Ethers have stronger intermolecular forces than hydrocarbons but much weaker intermolecular forces than alcohols thus boiling points are lowest for hydrocarbons, higher for ethers, and much higher for alcohols Low molecular weight ethers will dissolve in polar solvents (water) – high molecular weight ethers will dissolve in organic solvents but will not be water soluble (just like alcohols) Alkyl halides are classified as primary, secondary, or tertiary following the same rules as alcohols Alkyl halides contain a polar C-X bond and there are three lone pairs on the halide (F, Cl, Br, or I) Boiling and melting points for alkyl halides increase as the alkyl group size increases and as the halogen size increases Alkyl halides are incapable of hydrogen bonding and thus are always water insoluble! Nomenclature Alcohols: o Suffix = -ol o Longest carbon chain must contain the carbon bonded to the –OH group – this is given the lowest possible number o All other rules of nomenclature then apply o If the –OH group is bonded to a ring, that is automatically carbon #1 – do not put “1” in the name o What are diols and triols? Ethers: o Simple ethers are given common names Alphabetize the two alkyl groups and add the word “ether” ex: Ethyl methyl ether If two identical alkyl groups name as a “di-“ compound ex: Dimethyl ether o More complex ethers can be given alkoxy group names ex: 4-ethoxyoctane where 4 refers to the position on the octane chain that has the –OCH2CH3 (ethoxy group) bonded to it Phenyl group = Benzene ring attached to a parent compound Alkyl Halides: o Named as an alkane with a halogen substituent (fluorine fluoro-, chlorine chloro-, bromine bromo-, iodine iodo-) o Find parent chain that contains the carbon attached to the halogen o All other nomenclature rules apply o Common names may be used (ex: chloroethane = ethyl chloride) Recommended Textbook Practice Problems (All answers are in the back of the book): 14.29, 14.31, 14.33, 14.41, 14.43, 14.45, 14.47, 14.51 (a and b), 14.53 (skip e), 14.55, 14.57, 14.59, 14.61, 14.63, 14.65, 14.67, 14.69, 14.71, 14.73 Chapter 15 – Three-Dimensional Shape of Organic Compounds Definitions Superimposable Non-superimposable Chiral Achiral Stereocenter/chirality center Enantiomers Fischer Projections Plane-polarized light Optical Activity Polarimeter Dextrorotatory (D) Levorotatory (L) Specific Rotation Diastereomers Basic Concepts Recall the differences between constitutional isomers and stereoisomers What types of everyday objects are mirror images of each other? What makes a molecule have a chirality center (or a sterocenter)? Can you locate a chirality center in a molecule? A pair of enantiomers are isomers that are non-superimposable mirror images of one another Be able to draw a pair of enantiomers using the wedge diagrams and Fischer projections. What is plane polarized light and how is it used in the laboratory to differentiate a pair of optical isomers? What is the difference between a dextrorotatory (D) enantiomer and a levorotatory (L) enantiomer in an enantiomeric pair? When a compound has two or more chirality centers, some stereoisomers will be enantiomers of each other and some will be diasteromers of each other. Can you tell the difference? Recommended Textbook Practice Problems (All answers are in the back of the book): 15.23, 15.25, 15.27, 15.31, 15.33, 15.37, 15.39, 15.43, 15.45, 15.53, 15.55, 15.57, 15.61, 15.65, 15.67