Biology Vocabulary
advertisement

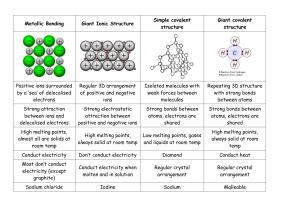
Biology Vocabulary Chemistry in Biology Mr.Adoff/Mrs.Naples Element-a substance which cannot be broken down into simpler substances Atom-the smallest particle in an element Nucleus-the center of an atom Proton-charged particle found inside the nucleus Electron-charged particle found outside the nucleus Neutron-particle found in the nucleus with no charge Atomic number-number found above an element on the periodic table Atomic mass-the number found below an element on the periodic table Compound-a substance that is made of two or more different elements bound together Molecule-a group of atoms bound together by covalent bonds Covalent bond-when two atoms share electrons Polar bond-occurs when the electrons of a bond are not shared equally Nonpolar bond-occurs when the electrons of a bond are shared equally Ionic bond-when two elements of opposite charge combine Ion-an atom that has acquired a positive or negative charge Reactant-substances that undergo the reaction Products-substances which are formed from a reaction Subscripts-the number of atoms of each element in a molecule Coefficient-the number before each chemical formula pH-refers to the hydrogen ion(H+) verses hydroxideion(H-) concentration in a solution Acid-a substance that has more H+ ions and has a pH below 7 Base-a substance that has more OH- ions and has a pH above 7 Ionization-when a nonionic compound is converted into ions