Name: Chemistry Exam Review
advertisement
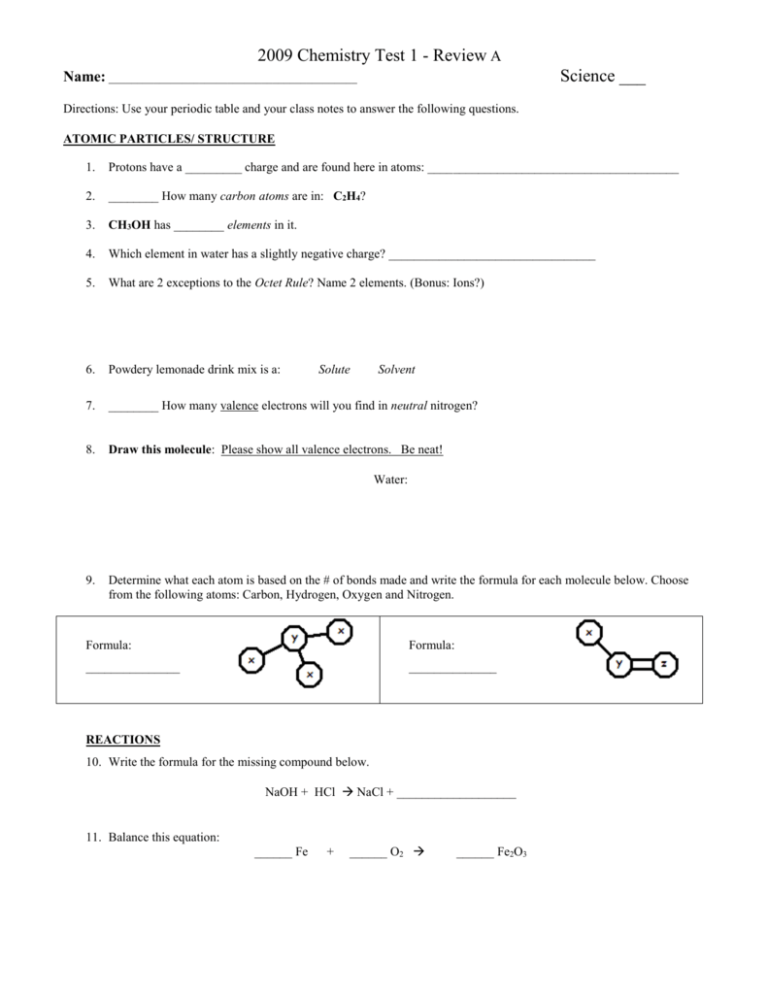
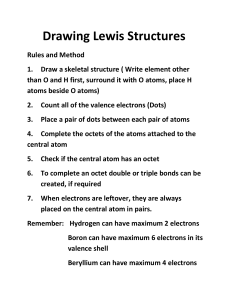
2009 Chemistry Test 1 - Review A Science ___ Name: ____________________________________________ Directions: Use your periodic table and your class notes to answer the following questions. ATOMIC PARTICLES/ STRUCTURE 1. Protons have a _________ charge and are found here in atoms: ________________________________________ 2. ________ How many carbon atoms are in: C2H4? 3. CH3OH has ________ elements in it. 4. Which element in water has a slightly negative charge? _________________________________ 5. What are 2 exceptions to the Octet Rule? Name 2 elements. (Bonus: Ions?) 6. Powdery lemonade drink mix is a: 7. ________ How many valence electrons will you find in neutral nitrogen? 8. Draw this molecule: Please show all valence electrons. Be neat! Solute Solvent Water: 9. Determine what each atom is based on the # of bonds made and write the formula for each molecule below. Choose from the following atoms: Carbon, Hydrogen, Oxygen and Nitrogen. Formula: Formula: _______________ ______________ REACTIONS 10. Write the formula for the missing compound below. NaOH + HCl NaCl + ___________________ 11. Balance this equation: ______ Fe + ______ O2 ______ Fe2O3 2009 Chemistry Test 1 - Review A 12. What is the result of the combustion of ethylene gas? You need to write the formulas for oxygen as well as the products of reaction. C2H4 + 13. What does it mean when matter is conserved during chemical reactions? Explain. 14. How many electrons can fit in the 2nd shell of an atom? Two Eight Ten 15. Could you burn a log on the surface of the moon? Why or why not? 16. What kind of change happens if ice melts? Chemical Physical 17. Name an element that will not react chemically with other atoms. __________________________________ 18. Describe how the properties of simple compounds, such as water, are different from the properties of the elements of which they are made. In your answer, explain the properties of the elements of water and how they differ. 19. List two examples of each type of classification in each box below: Element Compound Solution Colloid Suspension 20) Which 2 of the preceding types of matter are pure? Write “pure” in the boxes above. Heterogeneous