MIAPE_Quant_v1.0_SILAC_CNB
advertisement
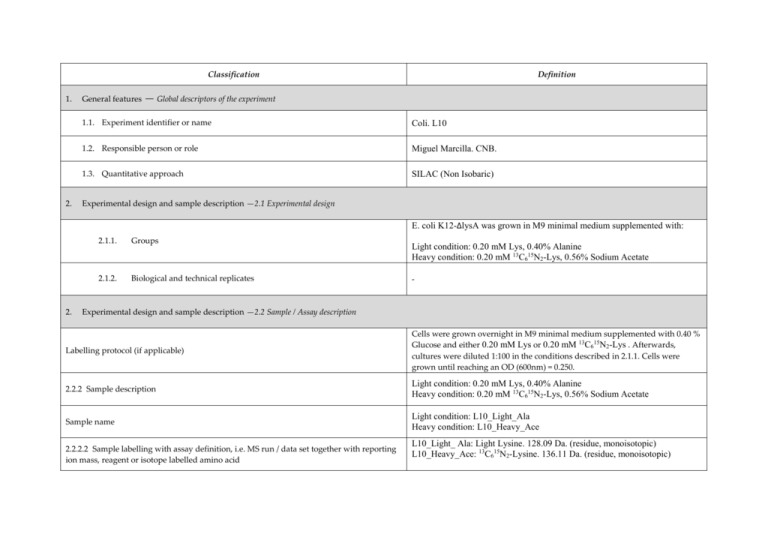
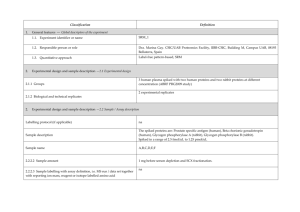
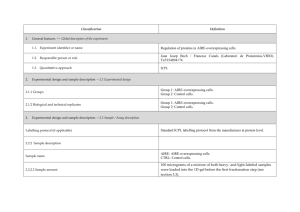
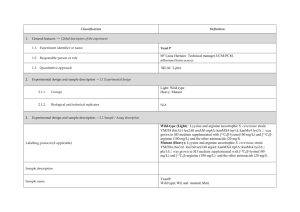
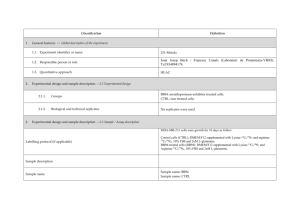
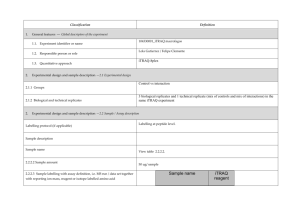
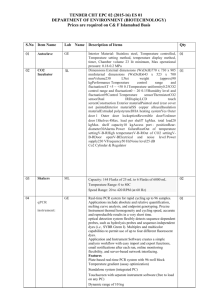
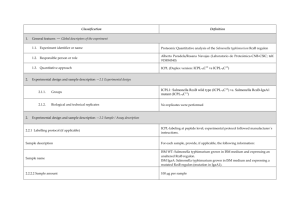
Classification 1. 2. Definition General features — Global descriptors of the experiment 1.1. Experiment identifier or name Coli. L10 1.2. Responsible person or role Miguel Marcilla. CNB. 1.3. Quantitative approach SILAC (Non Isobaric) Experimental design and sample description —2.1 Experimental design E. coli K12-∆lysA was grown in M9 minimal medium supplemented with: 2. 2.1.1. Groups 2.1.2. Biological and technical replicates Light condition: 0.20 mM Lys, 0.40% Alanine Heavy condition: 0.20 mM 13C615N2-Lys, 0.56% Sodium Acetate - Experimental design and sample description —2.2 Sample / Assay description Labelling protocol (if applicable) Cells were grown overnight in M9 minimal medium supplemented with 0.40 % Glucose and either 0.20 mM Lys or 0.20 mM 13C615N2-Lys . Afterwards, cultures were diluted 1:100 in the conditions described in 2.1.1. Cells were grown until reaching an OD (600nm) = 0.250. 2.2.2 Sample description Light condition: 0.20 mM Lys, 0.40% Alanine Heavy condition: 0.20 mM 13C615N2-Lys, 0.56% Sodium Acetate Sample name Light condition: L10_Light_Ala Heavy condition: L10_Heavy_Ace 2.2.2.2 Sample labelling with assay definition, i.e. MS run / data set together with reporting ion mass, reagent or isotope labelled amino acid L10_Light_ Ala: Light Lysine. 128.09 Da. (residue, monoisotopic) L10_Heavy_Ace: 13C615N2-Lysine. 136.11 Da. (residue, monoisotopic) 2.2.2.3 Sample amount 75 ug were loaded onto a 12% polyacrilamide gel 2.2.2.4 Replicates and/or groups - 2.2.3 Isotopic correction coefficients 13 2.2.4 Internal references - 3. 4. C615N2-Lysine Purity > 98% Input data — Description and reference of the dataset used for quantitative analysis: type, format and availability of the data. No actual values are requested here. 3.1. Input data type Full MS scans 3.2. Input data format Raw full traces (Bruker .yep) 3.3. Input data merging Sample was fractionated in a 12% polyacrilamide gel. 30 slices were excised and ≈ 1/3 of each slice was trypsin digested. Raw data were combined using the Analysis Combiner tool (Bruker) before quantitation. 3.4. Availability of the input data - Protocol —Description of the software and methods applied in the quantitative analysis (including transformation functions, aggregation functions and statistical calculations). 4.1. Quantification software name, version and manufacturer 4.2. Description of the selection and/or matching method of features, together with the description of the method of the primary extracted quantification values determination for each feature and/or peptide WARPLC 1.1 (Bruker) Quantitation based on Peak Intensity Mass Tolerance for Isotope pairs detection: 0.5 m/z units Retention time tolerance: 40 s Charge states: 1+, 2+, 3+ Smoothing: Activated Singlet defined as ratio>100 or ratio<0.01 4.3. Confidence filter of features or peptides prior to quantification 4.4. Description of data calculation and transformation methods 4.4.1. 4.4.2. Missing values imputation and outliers removal - Quantification values calculation and / or ratio determination from the Log2Ratio between the light and heavy monoisotopic signal intensities of each peptide passing the threshold described in 4.3. primary extracted quantification values 4.4.3. Replicate aggregation - 4.4.4. Normalization Median peptideLog2 ratio for all measurements forced to 0 4.4.5. Protein quantification values calculation and / or ratio determination from Only proteins with > 2 peptides were considered. Protein Log2ratio was defined as the average of the peptide Log2ratios of each protein group. the peptide quantification values 4.4.6. 5. Only peptides with MASCOT score >= 30.08 were considered. This threshold correlates with a FDR < 0.5% at the spectrum match level, < 1% at the peptide level and < 5% at the protein level. Provide, if applicable, the following information. Also state at which level (feature, peptide and/or protein) are performed and in which order: (mathematical formulas are allowed) Protocol specific corrections - 4.5. Description of methods for (statistical) estimation of correctness - 4.6. Calibration curves of standards - Resulting data —Provide the actual quantification values resulting from your quantification software together with their estimated confidence. Depending of the quantification technique or even of the quantification software, only some of the following items could be satisfied (e.g., for spectral counting, only quantification values at protein level can be provided) 5.1 Quantification values at peptide and/or feature level: Actual quantification values achieved for each peptide and/or, in case of feature-based quantification, for the corresponding features (mapped back from each peptide), together with their estimated confidence. 5.1.1 Primary extracted quantification values for each feature (e.g. area, height, etc.), with their statistical estimation of correctness http://proteo.cnb.csic.es/downloads/miape-quant/SILAC_CNB_5.1.1.xlsx 5.1.2 Quantification values for each peptide as a result of the aggregation of the values of the previous section (5.1.1), with their statistical estimation of correctness http://proteo.cnb.csic.es/downloads/miape-quant/SILAC_CNB_5.1.2.xlsx 5.2 Quantification values at protein level: Actual quantification values achieved for each protein and for each protein ambiguity group, together with the confidence in the quantification value. Basic / raw quantification values with statistical estimation of correctness - 5.2.2 Transformed / aggregated / combined quantification values of the proteins at group level, with their statistical estimation of correctness http://proteo.cnb.csic.es/downloads/miape-quant/SILAC_CNB_5.2.2.xlsx