Periodic Table Notes 2
advertisement

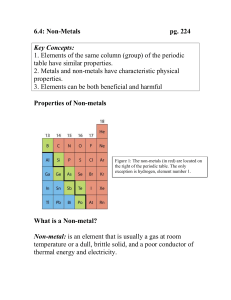
Topic #2: Elements, Compounds, & Chemical Reactions III. The Metals There are 85 elements classified as METALS. o The metals are located on the left side of the periodic table. o The metals make up the majority of the elements. o The metals are shiny, reflect light, and are good conductors of heat and electricity. o Gold and Silver are the best conductors, but Aluminum and Copper work just as well and are less expensive. IV. The Non-Metals There are 10 elements classified as NON-METALS. o The non-metals are located on the right side of the periodic table. o The non-metals have little or no luster and are usually dull in color. o The non-metals are brittle and are poor conductors of heat and electricity. o Many non-metals are gases at room temperature. V. The Metalloids There are 8 elements classified as METALLOIDS. o The metalloids are located along the zigzag line on the right side of the periodic table. o These elements have BOTH metal and non-metal properties. o The metalloids are mostly dull gray solids that are not good conductors of heat or electricity. Topic #2: Elements, Compounds, & Chemical Reactions VI. The Noble Gases (Inert Gases) There are 6 elements classified as NOBLE GASES. o The noble gases are located on the extreme right side of the periodic table. The noble gases are in Group 18. o All noble gases have the maximum number of electrons in their outer energy level (usually 8, except Helium which has just 2). This condition makes them very stable atoms. For this reason, they don’t usually combine with other elements to form compounds.