Metals, Non-Metals, Metalloids, and Noble Gases
advertisement

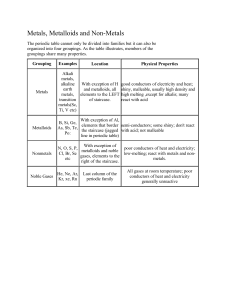
Cornell Notes 2-2 – Metals, Non-Metals, Metalloids, and Noble Gases October 22, 2015 - Page 51 and 53 • • • Most of the pure elements are solid at room temperature. Only 11 naturally occurring elements are a gas. Only 2 elements (Br and Hg) are liquid at room temperature. • On the big board up front, – solids are black – liquids are blue – gases are red – man-made elements are clear • Periodicity means properties repeat each period (row) of the periodic table. • • • • • • Most elements are either Metals or Non-metals Metals are on the left side of the periodic table. The ones at the bottom are also metals Most elements are metals Non-metals are the elements on the right side of the table. For example, all the “gas” elements are non-metals There are a few elements in the middle called metalloids. They have properties of both metals and non-metals. The staircase on your periodic table is the metal/non-metal boundary. Elements touching the staircase (except Al) are metalloids • • Notice that Hydrogen is a non-metal, even though it belongs to the Alkali Metals Group! It is NOT considered an Alkali Metal! • • • Electricity is the movement of electric charge, usually electrons. Some materials, like metals, allow electrons to flow easily through them. We call these materials electrical conductors. • • Like copper, most metals are good thermal conductors. That is one reason pots and pans are made of metal. • • Nonmetals make good insulators. An insulator is a material which slows down or stops the flow of either heat or electricity. • • Because metalloids are half-and-half, they are often good semiconductors. Silicon is a good example of a semiconductor which is why it is used to make computer chips! • • • The Noble Gases are the elements on the far right of the periodic table. They are the cool kids of the periodic table. Because they have complete electron, shells, they don’t form compounds with other elements. •