Electrophilic Aromatic Substitution
advertisement
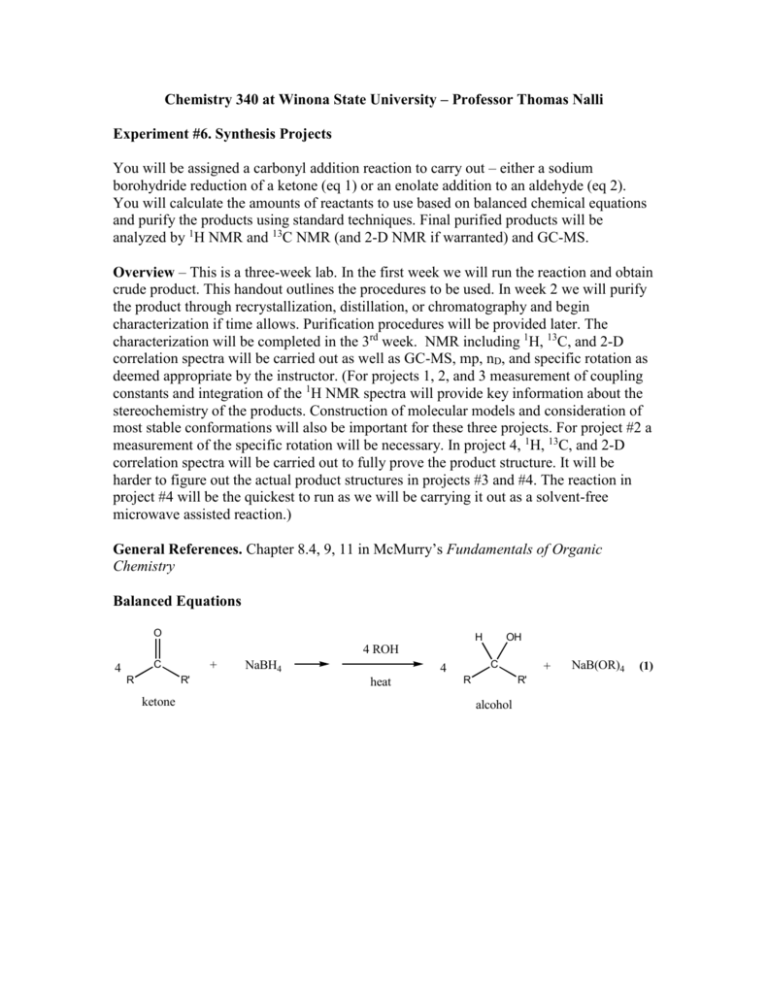
Chemistry 340 at Winona State University – Professor Thomas Nalli Experiment #6. Synthesis Projects You will be assigned a carbonyl addition reaction to carry out – either a sodium borohydride reduction of a ketone (eq 1) or an enolate addition to an aldehyde (eq 2). You will calculate the amounts of reactants to use based on balanced chemical equations and purify the products using standard techniques. Final purified products will be analyzed by 1H NMR and 13C NMR (and 2-D NMR if warranted) and GC-MS. Overview – This is a three-week lab. In the first week we will run the reaction and obtain crude product. This handout outlines the procedures to be used. In week 2 we will purify the product through recrystallization, distillation, or chromatography and begin characterization if time allows. Purification procedures will be provided later. The characterization will be completed in the 3rd week. NMR including 1H, 13C, and 2-D correlation spectra will be carried out as well as GC-MS, mp, nD, and specific rotation as deemed appropriate by the instructor. (For projects 1, 2, and 3 measurement of coupling constants and integration of the 1H NMR spectra will provide key information about the stereochemistry of the products. Construction of molecular models and consideration of most stable conformations will also be important for these three projects. For project #2 a measurement of the specific rotation will be necessary. In project 4, 1H, 13C, and 2-D correlation spectra will be carried out to fully prove the product structure. It will be harder to figure out the actual product structures in projects #3 and #4. The reaction in project #4 will be the quickest to run as we will be carrying it out as a solvent-free microwave assisted reaction.) General References. Chapter 8.4, 9, 11 in McMurry’s Fundamentals of Organic Chemistry Balanced Equations O H OH 4 ROH + C 4 R R' ketone NaBH4 C 4 heat R + R' alcohol NaB(OR)4 (1) O O C + C R O H aldehyde C R H H+ C R H C C O enolate C C C O O base (2) initial aldol product often reacts further CH C O Reaction Procedures Check your calculations with the instructor before beginning your reaction. Procedures for NaBH4 reductions – Projects 1 and 2 Project 1 – Reaction of 1,5-diphenyl-1,5-pentanedione with NaBH4: formation of a diol with two chiral carbons. Use 0.01 mol of the dione as the limiting reactant. Calculate the amount of NaBH4 to use based on the fact that it can donate all four of its hydrides (see eq 1). Also remember that the ketone here is a dione with two C=O groups to reduce. Use twice as much NaBH4 as is theoretically necessary so that there is a significant excess of this reactant. Use 10 mL of methanol as the solvent. Carry out the reaction using the general procedure below. Project 2 – Reaction of (R)-(+)-3-methylcyclohexanone with NaBH4: formation of a second chiral carbon. Use 0.01 mol of the cyclohexanone as the limiting reactant. Calculate the amount of NaBH4 to use based on the fact that it can donate all four of its hydrides (see eq 1). Use twice as much NaBH4 as is theoretically necessary so that there is a significant excess of this reactant. Use 5 mL of methanol as the solvent. Carry out the reaction using the general procedure below. General procedure. You can run the reaction in an appropriately sized Erlenmeyer flask or a round bottom flask if you prefer. Dissolve the ketone in methanol and cool on an ice bath. Carefully add the NaBH4 in portions to the stirring solution. When the vigorous reaction has ended allow the reaction to warm to room temperature over the course of about 15 min and then heat it on a boiling water bath (or hot plate set on low) for 5 min. After cooling for a few minutes add the solution to 10 mL of an ice/water mixture. If a solid forms then collect it by vacuum filtration. If not extract the solution in a separatory funnel with 2 x 5 mL of dichloromethane. Dry the organic extract over Na2SO4 and remove the solvent by rotary evaporation. Procedures for Projects 3 and 4 Project 3 – Reaction of acetone enolate with two equivalents of benzaldehyde: an aldol condensation that forms a diene with 3 possible stereoisomers. Use 0.05 mol of benzaldehyde. Calculate the amount of acetone to use based on the fact that it can form an enolate on both sides of the C=O and, thus react with two molecules of benzaldehyde. Mix the benzaldehyde and acetone together in an Erlenmeyer flask. Dissolve 5 g NaOH in a mixture of 50 mL H2O and 40 mL ethanol. Add half of the benzaldehyde/acetone solution. Allow the reaction to proceed for 15 minutes (with stirring or swirling) and then add the rest of the benzaldehyde solution. Allow the reaction to proceed for another 30 minutes and then collect the product by vacuum filtration. Wash the solid with 100 mL of H2O three times then press it as dry as possible and save for further purification next week. Project 4 – Reaction of malonic ester enolate with salicylaldehyde: synthesis of a coumarin ring via a Knoevenagel condensation. Use 0.1 mol of salicylaldehyde. Calculate the amount of malonic ester (diethyl malonate) to use based on a 1:1 stoichiometry figuring in a 10% excess of the ester. Mix the two reactants in a small beaker and add 0.2 g piperidine. (Piperidine functions as the base in the reaction – it reacts with diethyl malonate to form the enolate ion.) Place the beaker in the microwave oven and irradiate for 10 s at 10% power. Remove and immediately check the temperature of the solution. Repeat the microwave irradiation if necessary until the reaction mixture’s temperature reaches 130°C. After cooling to room temperature the crude solid can be recrystallized from ethanol/water to afford a pure final product.











