Author(s): MELO 3D Project Team, 2012 License
advertisement

Author(s): MELO 3D Project Team, 2012
License: Unless otherwise noted, this material is made available under the terms of the
Creative Commons Attribution – Share-Alike 3.0 License:
http://creativecommons.org/licenses/by-sa/2.0/
We have reviewed this material in accordance with U.S. Copyright Law and have tried to maximize your ability to use,
share, and adapt it. The attribution key found at the end of the module provides information about how you may share and
adapt this material.
Copyright holders of content included in this material should contact open.michigan@umich.edu with any questions,
corrections, or clarification regarding the use of content.
For more information about how to cite these materials visit http://open.umich.edu/education/about/terms-of-use.
Any medical information in this material is intended to inform and educate and is not a tool for self-diagnosis or a replacement
for medical evaluation, advice, diagnosis or treatment by a healthcare professional. Please speak to your physician if you have
questions about your medical condition.
Viewer discretion is advised: Some medical content is graphic and may not be suitable for all viewers.
Attribution Key
for more information see: http://open.umich.edu/wiki/AttributionPolicy
Use + Share + Adapt
{ Content the copyright holder, author, or law permits you to use, share and adapt. }
Public Domain – Government: Works that are produced by the U.S. Government. (17 USC § 105)
Public Domain – Expired: Works that are no longer protected due to an expired copyright term.
Public Domain – Self Dedicated: Works that a copyright holder has dedicated to the public domain.
Creative Commons – Zero Waiver
Creative Commons – Attribution License
Creative Commons – Attribution Share Alike License
Creative Commons – Attribution Noncommercial License
Creative Commons – Attribution Noncommercial Share Alike License
GNU – Free Documentation License
Make Your Own Assessment
{ Content Open.Michigan believes can be used, shared, and adapted because it is ineligible for copyright. }
Public Domain – Ineligible: Works that are ineligible for copyright protection in the U.S. (17 USC § 102(b)) *laws in
your jurisdiction may differ
{ Content Open.Michigan has used under a Fair Use determination. }
Fair Use: Use of works that is determined to be Fair consistent with the U.S. Copyright Act. (17 USC § 107) *laws in
your jurisdiction may differ
Our determination DOES NOT mean that all uses of this 3rd-party content are Fair Uses and we DO NOT guarantee
that your use of the content is Fair.
To use this content you should do your own independent analysis to determine whether or not your use will be Fair.
ch216sp12syllabus: Experiment 2
Experiment 2: Reduction of Organic Compounds
Introduction: Hydride based reducing agents LiAlH4 (lithium aluminum hydride) and NaBH4 (sodium
borohydride) react with ketones and aldehydes to produce a 1° or 2° alcohol product. Both reagents were
discovered by Schlesinger in the 1940s and are routinely used in organic synthesis. NaBH4 is a milder reducing
agent than LiAlH4 and can be used in protic solvents, such as ethanol. Conversely LiAlH4 must always be used in
aprotic solvents, such as tetrahydrofuran, and under extremely rigorous anhydrous (moisture free) conditions.
LiAlH4 also requires a separate acidic work up step where reduction with NaBH4 does not. For more on these
reagents see Eğe sections 14.4 and 21.3 B.
S c hlesinger, H.I.; Brown, H.C. et. al. J. Am. Chem. Soc. 1952, 75, 186.
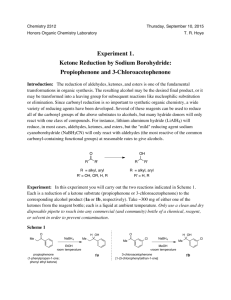
Reaction Scheme:
Note the stoichiometry of the reaction. Each molecule of NaBH4 can reduce up to 4 carbonyl groups since it
delivers 4 hydrides per NaBH4 molecule. Keeping that in mind, try drawing the balanced equation for this
reaction. Hint: one H on the alcohol comes from a hydride and the other comes from the acidic proton on
CH3CH2OH (ethanol).
Techniques used: Beilstein test, melting point, recrystallization, extraction & wash, drying agent, gravity &
vacuum filtration, thin layer chromatography, and infrared spectroscopy.
Objective: In part 1 you will reduce 9-fluorenone using the procedure described below. In part 2 you will reduce
an unknown ketone also using the method below. Your objective is to determine if the ketone unknown can be
reduced by NaBH4 to form an alcohol, to compare the two reactions (part 1 and part 2), and to determine the
identity of the unknown ketone.
Procedure:
Reagents:
9-fluorenone (part 1)
Unknown ketone (part 2)
Ethanol
NaBH4
In a dram vial, dissolve 0.1 g of 9-fluorenone (part 1) or unknown aromatic ketone (part 2) in 1 mL of 95%
ethanol, and cool the solution in ice (most ketones will produce a fine suspension). Add to this solution or
suspension 20 mg of sodium borohydride (a large excess). If you have a suspension the suspended ketone solid
will dissolve. The reaction mixture should warm up. After 15 minutes, add 1 mL of water, heat the solution to the
boiling point, dilute the hot solution with hot water (1-2 mL) to the point of saturation indicated by cloudiness.*
Collect the crystalline precipitates generated upon cooling the mixture to room temperature using vacuum
filtration. Recrystallize the reduction product. With the guidance of your GSI determine an appropriate solvent for
recrystallization.
*Note three of the unknown aromatic ketones should produce liquid products after reduction. If your
unknown product is a liquid the mixture will not become cloudy upon addition of 2 mL of water. In this
case you will perform a microscale extraction to isolate your product. Add 4-5 mL of diethyl ether to the
mixture and mix to allow the product to migrate into the organic layer. Transfer the ether layer into
another dram vial and wash with an equal volume of brine (saturated solution of NaCl). Pipet out the ether
layer and dry it over anhydrous magnesium sulfate. Remove the magnesium sulfate by gravity filtration
and evaporate the organic solvent by applying a stream of nitrogen gas.
Characterization: Determine the purity of the products in part 1 and 2, as well as the success of each reaction
using TLC. Collect the Infrared spectrum of each product and the unknown ketone starting material. Characterize
the starting materials and products of part 1 and 2 by m.p. Use the Beilstein test for halogens to rule out ketone
product 1 (the only unknown with halogen) from table 2-1 below. Use Reaxsys or SDBS to search for literature IR
spectra for the unknown ketone and its corresponding alcohol product for comparison to the spectra you obtain.
Post-lab Questions (write your answer at the end of your lab notebook pages for this experiment):
1.
Describe in your own words what happened to your ketone as NaBH4 is added. Why would a suspended
solid dissolve during this step?
2.
Why is 95 % ethanol used? Why not anhydrous ethanol?
3.
Compare the behavior of the unknown ketone with 9-fluorenone in the reduction reaction. How did the 2
reactions differ? How might the structural differences in the unknown ketone cause it to react differently than 9fluorenone?
4.
How does the Infrared spectrum of 9-fluorenone compare to its alcohol product? How does the spectrum
of your unknown alcohol product compare to that of the unknown ketone starting material?
Table 2-1